issue contents
June 2002 issue
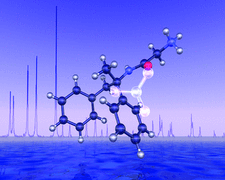
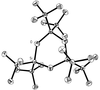
Cover illustration: The structure of remacemide nitrate (C17H21N2O+·NO3-) solved from powder diffraction data using a global optimization approach based on maximum likelihood. Only the remacemide molecule is optimized while the nitrate ion is treated as an unknown blur [Markvardsen, David & Shankland, Acta Cryst. (2002), A58. Submitted]. Image produced by Dr A. Florence, University of Strathclyde, Scotland, using POV-ray 3.1.
research papers






















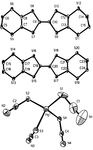
Chiral versus racemic building blocks in supramolecular chemistry: malate salts of organic diamines




addenda and errata






 journal menu
journal menu