issue contents
July 2014 issue
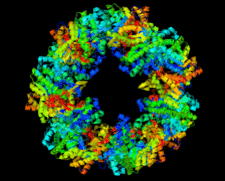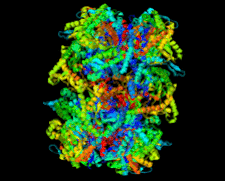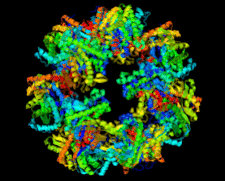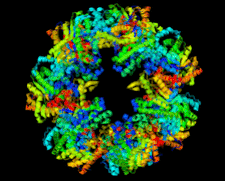
Cover illustration: Crystal structure of a new monomeric form of one of the ![[beta]](/logos/entities/beta_rmgif.gif) -glucosidases from Pyrococcus furiosus (mutant BGLPf-M6
-glucosidases from Pyrococcus furiosus (mutant BGLPf-M6![[Delta]](/logos/entities/Delta_rmgif.gif) C) in space group P1 (Nakabayashi et al., p. 854).
C) in space group P1 (Nakabayashi et al., p. 854).
IYCr crystallization series
Open  access
access
 access
accessThe rich history of crystallization and how that history influences current practices is described. The tremendous impact of crystallization screens on the field is discussed.
structural communications
Open  access
access
 access
accessA protein-engineering study revealed that the C-terminal domain of a thermostable β-glucosidase contributes to its polymeric state.
PDB reference: BGLPf, 3wq8
The crystal structure of the tetradecanucleotide d(CCCCGGTACCGGGG)2 as an A-DNA duplex and its solvent interactions are described.
PDB reference: d(CCCCGGTACCGGGG)2, 4okl
Open  access
access
 access
accessThe structure of VapB, a member of the Vap protein family that is involved in virulence of the bacterial pathogen R. equi, was determined by SAD phasing and reveals an eight-stranded antiparallel β-barrel similar to avidin, suggestive of a binding function. Made up of two Greek-key motifs, the topology of VapB is unusual or even unique.
PDB reference: R. equi VapB, 4cv7
S. typhimurium peptidyl-tRNA hydrolase was cloned, expressed, purified and crystallized. The crystals obtained diffracted to 1.6 Å resolution.
PDB reference: peptidyl-tRNA hydrolase, 4p7b
Open  access
access
 access
accessThe hyperthermostable endocellulase from P. furiosus was crystallized at pH 5.5. The new crystal form has symmetry consistent with space group C2 and exhibits a structure different from that of the protein crystallized at pH 9.0.
PDB reference: hyperthermophilic endocellulase, 3wq7
Crystallization of dienelactone hydrolase in an alternative space group results in more crystal contacts between neighbouring molecules and changes in the side-chain orientations at the new crystallographic interfaces.
A D-tagatose 3-epimerase-like protein from M. jannaschii has been crystallized and resolved at 2.64 Å resolution using the SIRAS method.
PDB reference: D-tagatose 3-epimerase-like protein, 3wqo
The structures of azide complexes of urate oxidase are reported.
crystallization communications
Here, the expression, purification, crystallization and preliminary crystallographic analysis of putative protein PA5088 from Pseudomonas aeruginosa are reported.
The PstS protein from Pseudomonas aeruginosa (PA) has a dual role in bacterial phosphate uptake and extracellular assembly of adhesion fibers. Here the subcloning, expression, isolation, crystallization and crystallographic analysis of native PA PstS are presented.
The crystal structure of zebrafish (D. rerio) complement 1qA globular domain (Dare-C1qAgD) was determined from X-ray diffraction data collected to 2.05 Å resolution. The crystal belonged to the orthorhombic space group P212121, with unit-cell parameters a = 50.347, b = 85.059, c = 95.560 Å.
A scorpion venom peptide from L. australasiae was crystallized in space group C2 and an S-SAD data set was collected to 1.9 Å resolution.
An Imp3–Mpp10 complex involved in yeast ribosome assembly has been crystallized.
An engineered version of glutamyl-tRNA synthetase from E. coli (double mutant K236E/E328A) has been crystallized.
Studies pertaining to the overexpression, purification, crystallization and preliminary X-ray work of AspB from M. tuberculosis are reported.
EhRabX3, a tandem GTPase from Entamoeba histolytica, was cloned, expressed, purified and crystallized. An X-ray diffraction data set was collected from a native crystal to 3.3 Å resolution. The crystal belonged to monoclinic space group C2.
The plasminogen binding protein glyceraldehyde-3-phosphate dehydrogenase from S. agalactiae was overexpressed, purified and crystallized, and its preliminary X-ray diffraction analysis is reported at 2.6 Å resolution.
Histamine dehydrogenase isolated from the newly identified halophilic archaeon N. gari BCC 24369 was purified and crystallized. The crystals belonged to the monoclinic space group C2, with unit-cell parameters a = 211.9, b = 58.6, c = 135.4 Å, β = 103.0°, and diffracted to a resolution of 2.4 Å.
The catalytic domain of a thermophilic GH16 β-1,3–1,4-glucanase from C. thermocellum has been crystallized and diffracted to a high resolution of 1.95 Å.
The C-terminal helical domain of GKAP was expressed, purified and crystallized in fusion with maltose-binding protein. Diffraction data were collected to 2.0 Å resolution.
The (S)-3-hydroxybutyryl-CoA dehydrogenase PaaH1 from R. eutropha (RePaaH1), an enzyme involved in n-butanol biosynthesis, has been crystallized, and X-ray diffraction data have been collected and analyzed.
EDR1 mediates resistance to biotic and antibiotic insults in A. thaliana. A catalytically inactive form of the catalytic domain was crystallized. The crystals belonged to space group P3221, contained two molecules per asymmetric unit and diffracted to 2.5 Å resolution.
The xylanase Xyn30D from P. barcinonensis was overexpressed, purified and crystallized, and its preliminary X-ray diffraction analysis is reported at 2.3 Å resolution.
Gos1 protein (Golgi SNAP receptor complex member 1) is involved in the SNAREs complexes which are the core machinery driving membrane fusion between cargo-carrying vesicles and their target membranes in the secretory and endocytic pathways in yeast. In this paper, the crystallization method of truncated versions of the Gos1 protein from Saccharomyces cerevisiae is reported and preliminary data on its X-ray analysis are provided.
The crystallization of three C-terminal fragments of the bacteriophage T4 protein gp34 is reported. Diffraction data have been obtained for three native crystal forms and two selenomethionine derivatives, one of which contained high-quality anomalous signal.
The UDP-N-acetylmuramoyl-tripeptide-D-alanyl-D-alanine ligase from A. baumannii has been crystallized and preliminary X-ray diffraction data have been collected.
The preliminary results of the crystallization and room-temperature diffraction of an inositol dehydrogenase are reported.
A putative nucleotide phosphohydrolase, YpgQ, from B. subtilis was expressed, purified and crystallized. The YpgQ crystal diffracted X-rays to a resolution of 2.3 Å.
The NqrA and NqrC subunits of the respiratory Na+-NQR complex were crystallized and analyzed by X-ray crystallography at 1.9 and 1.8 Å resolution, respectively.


 journal menu
journal menu


































![[publBio]](/logos/publbio.gif)