issue contents
June 2019 issue
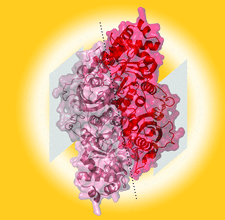
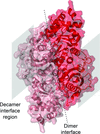
Cover illustration: Structure of a hyperthermostable dimeric archaeal Rubisco from Hyperthermus butylicus [Bundela et al. (2019), Acta Cryst. D75, 536-544]. Hbu-Rubisco is a monomer in the asymmetric unit, but the dimeric arrangement can be formed using symmetry operations, with the dashed line indicating the dimer interface. Unlike some other Rubiscos, Hbu-Rubisco does not assemble into decamers, but the location of the equivalent decameric interface is indicated by a grey box.
obituaries
Free 

Michael Rossmann, a pioneer in macromolecular and virus crystallography, is remembered.
feature articles
Open  access
access
 access
accessCM01 is a recently opened cryo-electron microscopy facility located at the European Synchrotron and is used by the ESRF's international community for structural biology.
research papers
While a decameric structure was previously thought to be essential for high thermal stability in archaeal Rubisco enzymes, a hyperthermophilic Rubisco enzyme that exists as a dimer is described.
PDB reference: dimeric archeal Rubisco from Hypothermus butylicus, 6hun
The structure of ligand-free spermidine N-acetyltransferase (SpeG) from Escherichia coli is described. Structural analysis was performed using X-ray crystallography and was combined with SEC-MALS, SAXS and negative-staining EM for characterization of the solution structure. In solution, SpeG from E. coli exhibits an oligomeric composition that is distinct from that of its homolog from Vibrio cholerae.
The crystal structure of the extracellular domain of HER2 is reported in complex with the humanized antibody HuA21-scFv, which has a distinct epitope and was developed by CDR grafting and affinity maturation on the basis of chA21 to reduce potential human anti-mouse immune response and to inhibit HER2-overexpressing cancer cells.
Open  access
access
 access
accessCMP-N-acetylneuraminate synthetase (CMAS) is a key enzyme in the sialic acid incorporation pathway and plays a crucial role in the virulence and survival of several pathogenic bacteria. Here, the structural and functional properties of CMAS from the pathogenic bacterium Vibrio cholerae are reported. Upon CDP binding, a partial domain closure is observed that was previously unreported in homologous structures. Kinetic studies reveal that the enzyme shows substrate promiscuity and can activate both Neu5Ac and Neu5Gc.
Coagulation factor XII (FXII) is a key initiator of the contact pathway and of kinin generation. Here, the first peptidomimetic complex structure of the activated protease domain βFXIIa is reported. The crystal structures of βFXIIa provide insight into substrate and inhibitor recognition by serine proteases.
Three high-resolution crystal structures of human phosphoserine phosphatase are described in complexes with phosphoserine, serine, phosphate and homocysteic acid, which are the substrate, products and substrate analogues for this enzyme. A dynamic study of the closing loop and an improved molecular mechanism (based on new key interactions) are also reported.
The crystal structure of the endoglucanase Ps_Cel5A from Pseudomonas stutzeri A1501 revealed aglycone-binding-site adaptations that are possibly linked to its transglycosylation activity.
book reviews
Free 



 journal menu
journal menu