Phenylphosphonic acid–4,4′-bipyridyl (1/1), (1), C6H7O3P.C10H8N2, triclinic, P1, a = 6.9026 (8), b = 9.7086 (9), c = 12.201 (2) Å, α = 77.138 (9), β = 74.345 (10), γ = 75.477 (8)°, with Z = 2, is a salt, C10H9N2+.[C6H5PO2(OH)]−, containing singly protonated 4,4′-bipyridyl cations: the cations and anions are linked by N—H O and C—H
O and C—H O hydrogen bonds in an
O hydrogen bonds in an 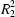 (7) motif and these aggregates are linked into centrosymmetric
(7) motif and these aggregates are linked into centrosymmetric 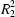 (8) dimers by O—H
(8) dimers by O—H O hydrogen bonds; the dimer units are linked into chains by C—H
O hydrogen bonds; the dimer units are linked into chains by C—H O hydrogen bonds. Phenylphosphonic acid–piperazine (2/1), (C6H7O3P)2.C4H10N2 (2), monoclinic, P21/n, a = 6.0042 (9), b = 19.746 (3), c = 8.651 (2) Å, β = 105.63 (2)°, with Z = 2, is a salt, C4H12N22+. [{C6H5PO2(OH)}−]2, containing doubly protonated piperazine: the anions are linked by O—H
O hydrogen bonds. Phenylphosphonic acid–piperazine (2/1), (C6H7O3P)2.C4H10N2 (2), monoclinic, P21/n, a = 6.0042 (9), b = 19.746 (3), c = 8.651 (2) Å, β = 105.63 (2)°, with Z = 2, is a salt, C4H12N22+. [{C6H5PO2(OH)}−]2, containing doubly protonated piperazine: the anions are linked by O—H O hydrogen bonds into centrosymmetric
O hydrogen bonds into centrosymmetric 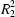 (8) dimers and these dimers are linked to the centrosymmetric cations by N—H
(8) dimers and these dimers are linked to the centrosymmetric cations by N—H O hydrogen bonds: each cation is hydrogen-bonded to four different anion dimers and each anion dimer is hydrogen-bonded to four different cations; the overall structure consists of two-dimensional sheets built from
O hydrogen bonds: each cation is hydrogen-bonded to four different anion dimers and each anion dimer is hydrogen-bonded to four different cations; the overall structure consists of two-dimensional sheets built from 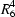 (16) and
(16) and 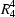 (18) rings. Phenylphosphonic acid–1,4-diazabicyclo[2.2.2]octane (2/1), (3), (C6H7O3P)2.C6H12N2, monoclinic, P21/n, a = 6.3607 (3), b = 21.8300 (11), c = 14.5965 (9) Å, β = 92.558 (6)°, with Z = 4, is a salt in which one nitrogen of the diamine is fully protonated and the other is partially protonated: the anionic components are linked into C(4) chains by O—H
(18) rings. Phenylphosphonic acid–1,4-diazabicyclo[2.2.2]octane (2/1), (3), (C6H7O3P)2.C6H12N2, monoclinic, P21/n, a = 6.3607 (3), b = 21.8300 (11), c = 14.5965 (9) Å, β = 92.558 (6)°, with Z = 4, is a salt in which one nitrogen of the diamine is fully protonated and the other is partially protonated: the anionic components are linked into C(4) chains by O—H O hydrogen bonds, and these chains are cross-linked via the diamines by means of N—H
O hydrogen bonds, and these chains are cross-linked via the diamines by means of N—H O and O—H
O and O—H N hydrogen bonds. The resulting sheets built from
N hydrogen bonds. The resulting sheets built from 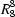 (34) rings are linked by C—H
(34) rings are linked by C—H O hydrogen bonds into a three-dimensional framework.
O hydrogen bonds into a three-dimensional framework.
 O and C—H
O and C—H O hydrogen bonds in an
O hydrogen bonds in an  O hydrogen bonds; the dimer units are linked into chains by C—H
O hydrogen bonds; the dimer units are linked into chains by C—H O hydrogen bonds. Phenylphosphonic acid–piperazine (2/1), (C6H7O3P)2.C4H10N2 (2), monoclinic, P21/n, a = 6.0042 (9), b = 19.746 (3), c = 8.651 (2) Å, β = 105.63 (2)°, with Z = 2, is a salt, C4H12N22+. [{C6H5PO2(OH)}−]2, containing doubly protonated piperazine: the anions are linked by O—H
O hydrogen bonds. Phenylphosphonic acid–piperazine (2/1), (C6H7O3P)2.C4H10N2 (2), monoclinic, P21/n, a = 6.0042 (9), b = 19.746 (3), c = 8.651 (2) Å, β = 105.63 (2)°, with Z = 2, is a salt, C4H12N22+. [{C6H5PO2(OH)}−]2, containing doubly protonated piperazine: the anions are linked by O—H O hydrogen bonds into centrosymmetric
O hydrogen bonds into centrosymmetric  O hydrogen bonds: each cation is hydrogen-bonded to four different anion dimers and each anion dimer is hydrogen-bonded to four different cations; the overall structure consists of two-dimensional sheets built from
O hydrogen bonds: each cation is hydrogen-bonded to four different anion dimers and each anion dimer is hydrogen-bonded to four different cations; the overall structure consists of two-dimensional sheets built from  O hydrogen bonds, and these chains are cross-linked via the diamines by means of N—H
O hydrogen bonds, and these chains are cross-linked via the diamines by means of N—H O and O—H
O and O—H N hydrogen bonds. The resulting sheets built from
N hydrogen bonds. The resulting sheets built from  O hydrogen bonds into a three-dimensional framework.
O hydrogen bonds into a three-dimensional framework.
 journal menu
journal menu











