issue contents
October 2013 issue
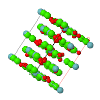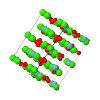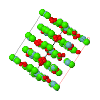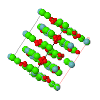
Cover illustration: Reduction of volumes of the voids in the crystal structures of DL-alanine and L-alanine on application of 6 GPa of pressure. Yellow voids are shown within the ball-and-stick structure models. The molecules as well as the topologies and symmetries of their packings are preserved over the whole pressure range of 0-6 GPa. See Fig. 4 in Tumanov & Boldyreva [(2012), Acta Cryst. B68, 412-423].
research papers
The bond-valence model used previously only for common ionic solids was adapted to (TM)6-chalcohalides, Mx(TM)6Ly (TM = transition metal in the cluster: Nb, Mo, W and Re; L = chalcogen and/or halogen ligands; M = counter-cation). This paper is devoted to the strains around M cations as one of the sources of the valence violations in cluster compounds.
The bond-valence model used previously only for common ionic solids was adapted to (TM)6-chalcohalides, Mx(TM)6Ly (TM = transition metal in the cluster: Nb, Mo, W and Re; L = the chalcogen and/or halogen ligands; M = counter-cation). This paper gives a detailed description of the matrix effect.
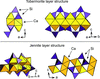
In this work we discuss the concept of tolerance factors for pyrochlores and related structures. We proposed a new form to calculate the tolerance factors for these structures and correlated the tolerance factor with some physical properties.
The volumes of 40 TeIVO6 coordination polyhedra studied are 10.3–23.7 Å3, compared with the 12.83 Å3 expected for a regular octahedron. The anions lie close to a spherical surface whose radius varies with the degree of lone-pair localization; 95% of the variation in polyhedron volume is due to a change in both sphere radius and the uniformity of oxygen distribution over the sphere.
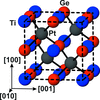
By the use of Rmerge factors on Friedel-difference intensities and the D-Patterson function, the structure of the intermetallic compound TiGePt is shown to be non-centrosymmetric.
Open  access
access
 access
accessThe structure of nanocrystalline calcium silicate hydrates (C-S-H) was studied using X-ray diffraction and literature data. It is proposed that C-S-H of Ca/Si ratio ranging between ∼ 0.6 and ∼ 1.7 can be described as nanocrystalline tobermorite affected by turbostratic disorder. The broadening and shift of the basal reflection positioned between ∼ 13.5 and ∼ 11.2 Å (depending on the Ca/Si ratio) arises from nanocrystallinity and possibly from an interstratification phenomenon.
Twins and polytypes in diamond and lonsdaleite nanoparticles were studied by high-resolution transmission electron microscopy, and the mechanisms of their appearance were proposed. Coherent and incoherent twin boundaries were observed in nanoparticles.
Download citation


Download citation


Monocationic amidate-bridged dimetal complexes reacted with dianionic group 6 metallate ions to form two-dimensional square-sheet or three-dimensional diamondoid structures depending on the alkyl group of the amidate ligands.
Download citation


Download citation


The three-dimensional structure of a hydrothermally synthesized new iron citrate compound, [Fe(H2cit)(H2O)]n, which is built up from the chains of corner-sharing FeO6 octahedra that are further connected through citrate anions, was solved by single-crystal X-ray diffraction. Thermal and magnetic properties of material were investigated by TG, HT-XRD, Mossbauer and magnetic susceptibility analyses.
Download citation


Download citation


A modulated molecular structure has been refined in (3 + 1)-dimensional superspace and also as two different Z′ = 17 commensurate approximations. The incommensurate modulation can be understood as relieving packing problems in several different directions.
B-IncStrDB reference: 8082ERT5uS
Download citation


Download citation


The crystal structures of two modifications of (1R,3S)-dimethyl 2-oxocyclohexane-1,3-dicarboxylate were solved simultaneously from a single crystal using X-ray diffraction.
Hydrogen bonds involving squaric acid and its monoanion are similar in length and strength to those made by carboxylic acid donors and carboxylate COO− acceptors, while hydrogen bonds accepted by the squarate dianion are somewhat stronger. The mono- and dianions have been added as new central groups to the Cambridge Crystallographic Data Centre's (CCDC) IsoStar knowledge base of intermolecular interactions.
book reviews
Free 



 journal menu
journal menu