issue contents
March 2001 issue
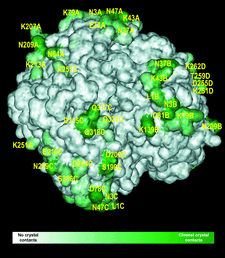
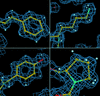
Cover illustration: Crystal contacts mapped on the solvent-accessible surface of E. coli L-asparaginase, an enzyme used for cancer therapy (p. 369).
research papers
The M148Q mutant of rusticyanin crystallizes with two molecules per asymmetric unit which shows a potential electron transfer route and redox partner docking site.
PDB reference: M148Q rusticyanin, 1e30
The crystal structures of a peptidyl-Lys metalloendopeptidase from G. frondosa were solved in four crystal forms. The structure belongs to a new `aspzincin` family with a novel active-site architecture.
Structures of two highly homologous bacterial L-asparaginases: a case of enantiomorphic space groups
The structures of the Y25F mutant of E. coli L-asparaginase and of native E. chrysanthemi L-asparaginase with quasi-enantiomorphic crystals obtained in the hexagonal space groups P6522 and P6122, respectively, are compared.
The structure of glycosylated fibroblast growth factor 9 has been determined at 2.6 Å resolution. FGF9 forms dimers with most of the receptor-binding residues buried in the dimer interface.
PDB reference: FGF9, 1g82
Anisotropic refinement at near-atomic resolution clarifies primary sequence discrepancies and reveals new structural details of the T. aurantiacus xylanase I.
PDB reference: xylanase I, 1fxm
The molecular structure of the ternary complex of S. cerevrisiae Nmt1p with a bound non-hydrolyzable myristoyl-CoA analog and a competitive peptidomimetic inhibitor has been solved to 2.9 Å.
For the metals Ca, Mg, Mn, Fe, Cu and Zn, geometry determined in high-resolution metalloprotein structures is compared with that in appropriate small-molecule complexes in the Cambridge Structural Database. Targets for refinement and for validation of distances and angles around the metal are proposed and unusual features of zinc carboxylate geometry highlighted.
Interferometric study on the development of concentration-depletion zones around protein crystals growing in microgravity and gelled on-ground experiments.
crystallization papers
The dihaem cytochrome NapB of H. influenzae was overexpressed, purified and crystallized. Native X-ray diffraction data were collected to 1.8 Å resolution using synchrotron radiation.
The crystallization and preliminary diffraction data analysis for the complex of an 15N-labelled mutant form of RTP and a symmetrical form of its DNA-binding site is reported.
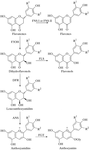
Crystals of anthocyanidin synthase from A. thaliana, a non-haem iron(II)-dependent dioxygenase involved in pigment production in higher plants, have been obtained both aerobically and anaerobically, and shown to diffract to 2.4 Å.
Recombinant P. furiosus prolidase, a dipeptide-specific proline aminopeptidase, has been crystallized in two forms. Laboratory and synchrotron data have been recorded.
Human betaine–homocysteine S-methyltransferase was cloned, expressed in E. coli, purified and crystallized. The crystals belong to the monoclinic space group C2, with unit-cell parameters a = 109.190, b = 91.319, c = 88.661 Å, β = 122.044°, and diffract to 2.9 Å.
The stoechiometric complex between the human RXRα ligand binding domain and 9-cis retinoic acid, its natural ligand, has been purified and crystallized in a tripartite mixture of sodium formate, glycerol and propane diol.
Crystallization and preliminary X-ray crystallographic analysis of RNase HIII from Bacillus subtilis
RNase HIII from B. subtilis has been overexpressed and crystallized. X-ray diffraction data have been collected to 2.8 Å resolution using synchrotron radiation.
A transcription-factor complex consisting of four different polypeptides and a DNA duplex has been assembled from purified components and crystallized. Diffraction data have been collected to 3.6 Å resolution.
NADPH 2-ketopropyl-coenzyme M oxidoreductase/carboxylase has been crystallized in the presence and absence of its reaction substrate and product and 1.65 Å resolution data have been collected.
The periplasmic binding protein ProX from E. coli has been crystallized with bound ligands (glycine betaine and proline betaine) and yields data to 1.9 Å resolution.
Glycerol-3-phosphate 1-acyltransferase from squash has been crystallized by the hanging-drop method of vapour diffusion using PEG 4000 as the precipitant. Preliminary data collected to 1.9 Å resolution have identified that these crystals are most likely to belong to space group P212121 with unit-cell parameters a = 61.1, b = 65.1, c= 103.3 Å and α = β = γ = 90°
Fructose-1,6-/sedoheptulose-1,7-bisphosphatase of Synechococcus PCC 7942 has been purified and crystallized. The crystals belong to the monoclinic space group P21 with unit-cell parameters a = 80.1, b = 84.2, c = 104.3 Å and β = 101.7°.
Polymethoxygalacturonase SX1 from T. penicillatum was crystallized and diffracted to 2.08 Å resolution. The crystals belong to the monoclinic space group C2, with unit-cell parameters a = 165.6, b = 61.0, c = 48.7 Å, β = 93.1°.
Forkhead-associated (FHA) domains of the cell-cycle checkpoint protein kinases Dun1p and Rad53p have been crystallized and X-ray diffraction data have been collected to 3.1 and 4.0 Å resolution, respectively.
The preliminary structure of an antibody (Fab41.4) that inhibits activating transcription factor 1 (ATF-1) from binding to a specific DNA sequence (CRE) is presented.
Orthogonal crystals of the human pyruvate dehydrogenase (E1) have been obtained. A native data set, complete to 2.5 Å has been collected.
A protein belonging to the Lrp/AsnC family from the hyperthermophilic archaeon Pyrococcus sp. OT3 was crystallized. In the crystal, a dimer is formed by two sets of four β-strands; the resultant dimers further associate.
short communications
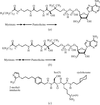
A 3.1 Å structure of HIV-1 protease in complex with a peptidomimetic inhibitor reveals the hydrogen-bonding pattern between active-site aspartic acids and the hydroxyethylamine transition-state analogue.
PDB reference: HIV-1–peptidomimetic inhibitor complex, 1fqx
book reviews
Free 

Free 

books received
Free 



 journal menu
journal menu