Buy article online - an online subscription or single-article purchase is required to access this article.
The hydrogen-bond acceptor ability of divalent sulfur in Y—S—Z systems, Y, Z= C, N, O or S, and the donor ability of thiol S—H have been studied using crystallographic data retrieved from the Cambridge Structural Database. Of 1811 Y—S—Z substructures that co-occur with N—H or O—H donors, only 86 (4.75%) form S H—N,O bonds within S
H—N,O bonds within S H < 2.9 Å. In dialkylthioethers, the frequency of S
H < 2.9 Å. In dialkylthioethers, the frequency of S H bond formation is 6.24%, but drops below 3% when the alkyl groups are successively replaced by Csp2 centres. This parallels an increasing
H bond formation is 6.24%, but drops below 3% when the alkyl groups are successively replaced by Csp2 centres. This parallels an increasing 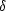 -positivity of S as calculated using ab initio methods. A similar frequency trend is observed for O
-positivity of S as calculated using ab initio methods. A similar frequency trend is observed for O H—N,O bond formation by analogous oxyethers. Mean intermolecular >S
H—N,O bond formation by analogous oxyethers. Mean intermolecular >S H distances for O—H [2.67 (3) Å] and N—H [2.75 (2) Å] donors (with H positions normalized to neutron values) are ca 0.25 Å longer than in C=S
H distances for O—H [2.67 (3) Å] and N—H [2.75 (2) Å] donors (with H positions normalized to neutron values) are ca 0.25 Å longer than in C=S H—N,O systems, indicative of very weak hydrogen bonding to >S. Intramolecular >S
H—N,O systems, indicative of very weak hydrogen bonding to >S. Intramolecular >S H are slightly more frequent (8.56%), with S
H are slightly more frequent (8.56%), with S H slightly shorter than for the intermolecular case. In contrast, 26 (70.3%) out of 37 S—H donors that co-occur with suitable acceptors form X
H slightly shorter than for the intermolecular case. In contrast, 26 (70.3%) out of 37 S—H donors that co-occur with suitable acceptors form X H—S bonds. The C=O
H—S bonds. The C=O H—S system is predominant with a mean O
H—S system is predominant with a mean O H distance of 2.34 (4) Å, considerably longer (weaker) than in C=O
H distance of 2.34 (4) Å, considerably longer (weaker) than in C=O H—O systems.
H—O systems.
 H—N,O bonds within S
H—N,O bonds within S H < 2.9 Å. In dialkylthioethers, the frequency of S
H < 2.9 Å. In dialkylthioethers, the frequency of S H bond formation is 6.24%, but drops below 3% when the alkyl groups are successively replaced by Csp2 centres. This parallels an increasing
H bond formation is 6.24%, but drops below 3% when the alkyl groups are successively replaced by Csp2 centres. This parallels an increasing  H—N,O bond formation by analogous oxyethers. Mean intermolecular >S
H—N,O bond formation by analogous oxyethers. Mean intermolecular >S H distances for O—H [2.67 (3) Å] and N—H [2.75 (2) Å] donors (with H positions normalized to neutron values) are ca 0.25 Å longer than in C=S
H distances for O—H [2.67 (3) Å] and N—H [2.75 (2) Å] donors (with H positions normalized to neutron values) are ca 0.25 Å longer than in C=S H—N,O systems, indicative of very weak hydrogen bonding to >S. Intramolecular >S
H—N,O systems, indicative of very weak hydrogen bonding to >S. Intramolecular >S H are slightly more frequent (8.56%), with S
H are slightly more frequent (8.56%), with S H slightly shorter than for the intermolecular case. In contrast, 26 (70.3%) out of 37 S—H donors that co-occur with suitable acceptors form X
H slightly shorter than for the intermolecular case. In contrast, 26 (70.3%) out of 37 S—H donors that co-occur with suitable acceptors form X H—S bonds. The C=O
H—S bonds. The C=O H—S system is predominant with a mean O
H—S system is predominant with a mean O H distance of 2.34 (4) Å, considerably longer (weaker) than in C=O
H distance of 2.34 (4) Å, considerably longer (weaker) than in C=O H—O systems.
H—O systems.
 journal menu
journal menu









 Subscribe to Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials
Subscribe to Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials


