The title compound, [Ti
2Cl
6(C
2H
6N)
2(C
2H
7N)
2], is a binuclear octahedral complex lying about an inversion centre. There are four different chloride environments, two terminal [Ti—Cl = 2.2847 (5) and 2.3371 (5) Å] and two bridging [Ti—Cl = 2.4414 (5) and 2.6759 (5) Å], with the Ti—Cl distances being strongly influenced by both the ligand
trans to the chloride and whether or not the chloride anion is bridging between the two Ti
IV centres. The compound forms a two-dimensional network in the solid state, with weak intermolecular C—H

Cl interactions giving rise to a planar network in the (10
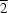
) plane.
Supporting information
CCDC reference: 838133
A solution of AsCl3 (0.2 ml, 2.37 mmol) in diethyl ether (20 ml) was added
dropwise to a solution of Ti(NMe2)4 (0.6 ml, 2.53 mmol) in diethyl ether
(20 ml). The reaction was stirred for 48 h and then the solvents were removed
under vacuum, affording a dark-green solid (yield 93%). Crystallization
occurred by layering a concentrated CH2Cl2 solution of (II) with hexane.
Spectroscopic analysis: 1H NMR (C6D6, 400 MHz, 298 K, δ, p.p.m.): 2.5
(s), 2.2 (s). Analysis, calculated for C4H13Cl3N2Ti: C
19.74, H 5.38, N 11.51%; found: C 19.57, H 5.51, N 11.62%.
All H atoms were clearly visible in difference maps and were refined using a
riding model. The methyl H atoms were constrained to an ideal geometry, with
C—H = 0.98 Å and Uiso(H) = 1.5Ueq(C), but were allowed
to rotate freely about the adjacent C—N bonds. Atom H2 bonded to N2 was
placed in a geometrically idealized position and constrained to ride, with
N—H = 0.93 Å and Uiso(H) = 1.2Ueq(N).
Data collection: COLLECT (Nonius, 1998); cell refinement: DENZO (Otwinowski & Minor, 1997) and COLLECT (Nonius, 1998); data reduction: DENZO (Otwinowski & Minor, 1997) and COLLECT (Nonius, 1998); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008); molecular graphics: ORTEP-3 (Farrugia, 1997) and Mercury (Macrae et al.,
2006); software used to prepare material for publication: PLATON (Spek, 2009), WinGX (Farrugia, 1999) and enCIFer (Allen
et al., 2004).
Di-µ-chlorido-bis[dichloridobis(methylamido-
κN)bis(methylamine-
κN)titanium(IV)]
top
Crystal data top
| [Ti2Cl6(C2H6N)2(C2H7N)2] | F(000) = 496 |
| Mr = 486.83 | Dx = 1.601 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 11855 reflections |
| a = 10.7088 (4) Å | θ = 2.9–27.5° |
| b = 6.7143 (3) Å | µ = 1.58 mm−1 |
| c = 15.7006 (5) Å | T = 120 K |
| β = 116.515 (2)° | Cut block, green |
| V = 1010.16 (7) Å3 | 0.16 × 0.10 × 0.08 mm |
| Z = 2 | |
Data collection top
Bruker Nonius APEXII CCD camera on κ-goniostat
diffractometer | 2317 independent reflections |
| Radiation source: Bruker-Nonius FR591 rotating anode | 2092 reflections with I > 2σ(I) |
| 10cm confocal mirrors monochromator | Rint = 0.039 |
| Detector resolution: 4096x4096pixels / 62x62mm pixels mm-1 | θmax = 27.5°, θmin = 3.6° |
| ϕ and ω scans | h = −13→13 |
Absorption correction: multi-scan
(SADABS; Sheldrick, 2007) | k = −8→8 |
| Tmin = 0.786, Tmax = 0.884 | l = −20→20 |
| 9954 measured reflections | |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.028 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.062 | H-atom parameters constrained |
| S = 1.12 | w = 1/[σ2(Fo2) + (0.P)2 + 1.0541P]
where P = (Fo2 + 2Fc2)/3 |
| 2317 reflections | (Δ/σ)max = 0.001 |
| 95 parameters | Δρmax = 0.32 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
Crystal data top
| [Ti2Cl6(C2H6N)2(C2H7N)2] | V = 1010.16 (7) Å3 |
| Mr = 486.83 | Z = 2 |
| Monoclinic, P21/c | Mo Kα radiation |
| a = 10.7088 (4) Å | µ = 1.58 mm−1 |
| b = 6.7143 (3) Å | T = 120 K |
| c = 15.7006 (5) Å | 0.16 × 0.10 × 0.08 mm |
| β = 116.515 (2)° | |
Data collection top
Bruker Nonius APEXII CCD camera on κ-goniostat
diffractometer | 2317 independent reflections |
Absorption correction: multi-scan
(SADABS; Sheldrick, 2007) | 2092 reflections with I > 2σ(I) |
| Tmin = 0.786, Tmax = 0.884 | Rint = 0.039 |
| 9954 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.028 | 0 restraints |
| wR(F2) = 0.062 | H-atom parameters constrained |
| S = 1.12 | Δρmax = 0.32 e Å−3 |
| 2317 reflections | Δρmin = −0.37 e Å−3 |
| 95 parameters | |
Special details top
Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell s.u.'s are taken
into account individually in the estimation of s.u.'s in distances, angles
and torsion angles; correlations between s.u.'s in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
Refinement. Refinement of F2 against ALL reflections. The weighted R-factor
wR and goodness of fit S are based on F2, conventional
R-factors R are based on F, with F set to zero for
negative F2. The threshold expression of F2 >
σ(F2) is used only for calculating R-factors(gt) etc.
and is not relevant to the choice of reflections for refinement.
R-factors based on F2 are statistically about twice as large
as those based on F, and R-factors based on ALL data will be
even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| Ti1 | 0.29532 (3) | 0.51821 (5) | 0.44300 (2) | 0.01379 (9) | |
| Cl1 | 0.29281 (5) | 0.37857 (7) | 0.30590 (3) | 0.02089 (12) | |
| Cl2 | 0.49136 (4) | 0.72771 (7) | 0.45984 (3) | 0.01622 (11) | |
| Cl3 | 0.15258 (5) | 0.28600 (7) | 0.46057 (3) | 0.02059 (12) | |
| N1 | 0.15872 (15) | 0.7093 (2) | 0.38217 (11) | 0.0171 (3) | |
| N2 | 0.35993 (16) | 0.6275 (2) | 0.59128 (11) | 0.0168 (3) | |
| H2 | 0.4567 | 0.6317 | 0.6170 | 0.020* | |
| C1 | 0.03618 (19) | 0.7453 (3) | 0.39827 (15) | 0.0233 (4) | |
| H1A | −0.0485 | 0.7121 | 0.3405 | 0.035* | |
| H1B | 0.0334 | 0.8859 | 0.4141 | 0.035* | |
| H1C | 0.0415 | 0.6619 | 0.4510 | 0.035* | |
| C2 | 0.1584 (2) | 0.8385 (3) | 0.30680 (15) | 0.0264 (4) | |
| H2A | 0.1694 | 0.9775 | 0.3280 | 0.040* | |
| H2B | 0.0698 | 0.8230 | 0.2493 | 0.040* | |
| H2C | 0.2357 | 0.8012 | 0.2927 | 0.040* | |
| C3 | 0.3221 (2) | 0.8364 (3) | 0.59991 (15) | 0.0262 (4) | |
| H3A | 0.3748 | 0.8809 | 0.6660 | 0.039* | |
| H3B | 0.2219 | 0.8446 | 0.5814 | 0.039* | |
| H3C | 0.3447 | 0.9218 | 0.5581 | 0.039* | |
| C4 | 0.3351 (2) | 0.5005 (3) | 0.65953 (14) | 0.0257 (5) | |
| H4A | 0.2348 | 0.4951 | 0.6410 | 0.038* | |
| H4B | 0.3842 | 0.5569 | 0.7236 | 0.038* | |
| H4C | 0.3699 | 0.3658 | 0.6590 | 0.038* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| Ti1 | 0.01305 (16) | 0.01539 (17) | 0.01466 (17) | −0.00119 (12) | 0.00773 (13) | −0.00037 (13) |
| Cl1 | 0.0188 (2) | 0.0277 (3) | 0.0169 (2) | −0.00273 (18) | 0.00864 (18) | −0.00555 (18) |
| Cl2 | 0.0154 (2) | 0.0148 (2) | 0.0207 (2) | −0.00089 (16) | 0.01006 (18) | 0.00132 (17) |
| Cl3 | 0.0193 (2) | 0.0187 (2) | 0.0270 (3) | −0.00443 (17) | 0.01321 (19) | −0.00021 (18) |
| N1 | 0.0145 (7) | 0.0186 (8) | 0.0181 (8) | −0.0005 (6) | 0.0072 (6) | 0.0014 (6) |
| N2 | 0.0143 (7) | 0.0197 (8) | 0.0179 (8) | 0.0004 (6) | 0.0086 (6) | −0.0008 (6) |
| C1 | 0.0165 (9) | 0.0240 (10) | 0.0297 (11) | 0.0028 (8) | 0.0107 (8) | 0.0013 (9) |
| C2 | 0.0269 (10) | 0.0268 (11) | 0.0262 (11) | 0.0026 (9) | 0.0125 (9) | 0.0075 (9) |
| C3 | 0.0304 (11) | 0.0225 (10) | 0.0251 (11) | 0.0035 (9) | 0.0119 (9) | −0.0060 (9) |
| C4 | 0.0310 (11) | 0.0327 (12) | 0.0192 (10) | −0.0034 (9) | 0.0166 (9) | 0.0012 (9) |
Geometric parameters (Å, º) top
| Ti1—N1 | 1.8576 (16) | C1—H1A | 0.9800 |
| Ti1—N2 | 2.2358 (16) | C1—H1B | 0.9800 |
| Ti1—Cl3 | 2.2847 (5) | C1—H1C | 0.9800 |
| Ti1—Cl1 | 2.3371 (5) | C2—H2A | 0.9800 |
| Ti1—Cl2 | 2.4414 (5) | C2—H2B | 0.9800 |
| Ti1—Cl2i | 2.6759 (5) | C2—H2C | 0.9800 |
| Cl2—Ti1i | 2.6759 (5) | C3—H3A | 0.9800 |
| N1—C1 | 1.463 (2) | C3—H3B | 0.9800 |
| N1—C2 | 1.466 (2) | C3—H3C | 0.9800 |
| N2—C3 | 1.483 (2) | C4—H4A | 0.9800 |
| N2—C4 | 1.483 (2) | C4—H4B | 0.9800 |
| N2—H2 | 0.9300 | C4—H4C | 0.9800 |
| | | |
| N1—Ti1—N2 | 96.62 (6) | N1—C1—H1A | 109.5 |
| N1—Ti1—Cl3 | 96.76 (5) | N1—C1—H1B | 109.5 |
| N2—Ti1—Cl3 | 90.63 (4) | H1A—C1—H1B | 109.5 |
| N1—Ti1—Cl1 | 96.95 (5) | N1—C1—H1C | 109.5 |
| N2—Ti1—Cl1 | 164.09 (4) | H1A—C1—H1C | 109.5 |
| Cl3—Ti1—Cl1 | 95.98 (2) | H1B—C1—H1C | 109.5 |
| N1—Ti1—Cl2 | 95.71 (5) | N1—C2—H2A | 109.5 |
| N2—Ti1—Cl2 | 81.19 (4) | N1—C2—H2B | 109.5 |
| Cl3—Ti1—Cl2 | 165.80 (2) | H2A—C2—H2B | 109.5 |
| Cl1—Ti1—Cl2 | 89.233 (19) | N1—C2—H2C | 109.5 |
| N1—Ti1—Cl2i | 174.37 (5) | H2A—C2—H2C | 109.5 |
| N2—Ti1—Cl2i | 79.65 (4) | H2B—C2—H2C | 109.5 |
| Cl3—Ti1—Cl2i | 87.537 (19) | N2—C3—H3A | 109.5 |
| Cl1—Ti1—Cl2i | 86.181 (18) | N2—C3—H3B | 109.5 |
| Cl2—Ti1—Cl2i | 79.619 (18) | H3A—C3—H3B | 109.5 |
| Ti1—Cl2—Ti1i | 100.381 (18) | N2—C3—H3C | 109.5 |
| C1—N1—C2 | 111.26 (15) | H3A—C3—H3C | 109.5 |
| C1—N1—Ti1 | 125.90 (13) | H3B—C3—H3C | 109.5 |
| C2—N1—Ti1 | 122.77 (13) | N2—C4—H4A | 109.5 |
| C3—N2—C4 | 109.48 (15) | N2—C4—H4B | 109.5 |
| C3—N2—Ti1 | 115.81 (12) | H4A—C4—H4B | 109.5 |
| C4—N2—Ti1 | 119.46 (12) | N2—C4—H4C | 109.5 |
| C3—N2—H2 | 103.2 | H4A—C4—H4C | 109.5 |
| C4—N2—H2 | 103.2 | H4B—C4—H4C | 109.5 |
| Ti1—N2—H2 | 103.2 | | |
| | | |
| N1—Ti1—Cl2—Ti1i | 176.84 (5) | Cl2—Ti1—N1—C2 | 42.68 (15) |
| N2—Ti1—Cl2—Ti1i | 81.00 (4) | N1—Ti1—N2—C3 | −19.88 (14) |
| Cl3—Ti1—Cl2—Ti1i | 25.58 (9) | Cl3—Ti1—N2—C3 | −116.75 (13) |
| Cl1—Ti1—Cl2—Ti1i | −86.258 (19) | Cl1—Ti1—N2—C3 | 128.50 (16) |
| Cl2i—Ti1—Cl2—Ti1i | 0.0 | Cl2—Ti1—N2—C3 | 74.90 (13) |
| N2—Ti1—N1—C1 | −58.77 (16) | Cl2i—Ti1—N2—C3 | 155.87 (13) |
| Cl3—Ti1—N1—C1 | 32.65 (16) | N1—Ti1—N2—C4 | 114.43 (14) |
| Cl1—Ti1—N1—C1 | 129.55 (15) | Cl3—Ti1—N2—C4 | 17.55 (13) |
| Cl2—Ti1—N1—C1 | −140.53 (15) | Cl1—Ti1—N2—C4 | −97.2 (2) |
| N2—Ti1—N1—C2 | 124.43 (15) | Cl2—Ti1—N2—C4 | −150.79 (14) |
| Cl3—Ti1—N1—C2 | −144.15 (15) | Cl2i—Ti1—N2—C4 | −69.83 (13) |
| Cl1—Ti1—N1—C2 | −47.25 (15) | | |
| Symmetry code: (i) −x+1, −y+1, −z+1. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···Cl1i | 0.93 | 2.40 | 3.3282 (16) | 175 |
| C1—H1B···Cl3ii | 0.98 | 2.92 | 3.822 (2) | 153 |
| C1—H1A···Cl1iii | 0.98 | 2.83 | 3.661 (2) | 143 |
| Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x, y+1, z; (iii) −x, y+1/2, −z+1/2. |
Experimental details
| Crystal data |
| Chemical formula | [Ti2Cl6(C2H6N)2(C2H7N)2] |
| Mr | 486.83 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 120 |
| a, b, c (Å) | 10.7088 (4), 6.7143 (3), 15.7006 (5) |
| β (°) | 116.515 (2) |
| V (Å3) | 1010.16 (7) |
| Z | 2 |
| Radiation type | Mo Kα |
| µ (mm−1) | 1.58 |
| Crystal size (mm) | 0.16 × 0.10 × 0.08 |
| |
| Data collection |
| Diffractometer | Bruker Nonius APEXII CCD camera on κ-goniostat
diffractometer |
| Absorption correction | Multi-scan
(SADABS; Sheldrick, 2007) |
| Tmin, Tmax | 0.786, 0.884 |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 9954, 2317, 2092 |
| Rint | 0.039 |
| (sin θ/λ)max (Å−1) | 0.650 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.028, 0.062, 1.12 |
| No. of reflections | 2317 |
| No. of parameters | 95 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.32, −0.37 |
Selected geometric parameters (Å, º) top| Ti1—N1 | 1.8576 (16) | Ti1—Cl1 | 2.3371 (5) |
| Ti1—N2 | 2.2358 (16) | Ti1—Cl2 | 2.4414 (5) |
| Ti1—Cl3 | 2.2847 (5) | Ti1—Cl2i | 2.6759 (5) |
| | | |
| N2—Ti1—Cl1 | 164.09 (4) | N1—Ti1—Cl2i | 174.37 (5) |
| Cl3—Ti1—Cl2 | 165.80 (2) | | |
| Symmetry code: (i) −x+1, −y+1, −z+1. |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···Cl1i | 0.93 | 2.40 | 3.3282 (16) | 175 |
| C1—H1B···Cl3ii | 0.98 | 2.92 | 3.822 (2) | 153 |
| C1—H1A···Cl1iii | 0.98 | 2.83 | 3.661 (2) | 143 |
| Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x, y+1, z; (iii) −x, y+1/2, −z+1/2. |
 Cl interactions giving rise to a planar network in the (10
Cl interactions giving rise to a planar network in the (10
 journal menu
journal menu








![[Figure 1]](https://journals.iucr.org/c/issues/2011/07/00/fg3220/fg3220fig1thm.gif)
![[Figure 2]](https://journals.iucr.org/c/issues/2011/07/00/fg3220/fg3220fig2thm.gif)




Chemical vapour deposition (CVD) is a well used technique for depositing thin films of materials (Jones & Hitchman, 2009). Industrially, films of transition metal pnictide materials are deposited using volatile metal and pnictogen precursors, but these precursors are often toxic, flammable and generally difficult to handle. `Single-source' precursors are compounds which contain a direct M—Pn bond (Pn = pnictogen) and they are usually much less toxic and much easier to handle (Cowley & Jones, 1994; Carmalt & Basharat, 2007).
As a part of recent work into the synthesis of molecular precursors for the deposition of transition metal pnictide thin films (Potts et al., 2009; Thomas, Blackman et al., 2010; Thomas et al., 2011), we recently reported an unusual ligand-exchange reaction between TiCl4 and As(NMe2)3 (Thomas, Pugh et al., 2010). The reaction of one equivalent of As(NMe2)3 with TiCl4 did not afford the 1:1 adduct [TiCl4{As(NMe2)3}]. Instead, the compound [TiCl3(NMe2)(µ-NMe2)2AsCl], (I), was isolated as dark-green crystals. Structural characterization confirmed that an exchange of chloride and dimethylamide ligands had taken place.
Despite the fact that (I) was largely unsuitable as a single-source precursor to thin films of TiAs (owing to the lack of a direct Ti—As bond), further investigations into this unusual exchange were carried out. In an attempt to determine whether the exchange would still occur if the ligands on the metals were reversed, one equivalent of [Ti(NMe2)4] was reacted with AsCl3. The title compound, (II), also a dark-green solid, was isolated from the reaction.
Compound (II) is a binuclear slightly distorted octahedral complex lying about an inversion centre, with two 14-electron TiIV centres. The bridging chloride ligand datively bonds trans to the dimethylamide group, with the four ligands cis to the dimethylamide group showing a slight repulsion from the strongly electronegative group (Fig. 1). The Ti2Cl2 ring at the heart of the molecule is planar, with atom N1 departing from this plane by 0.102 (2) Å.
The Ti—N bond lengths in (II) give a strong indication of the presence of two different types of N-containing ligand within the complex (Table 1). The large difference between the Ti—N1 and Ti—N2 bond lengths is indicative of the presence of anionic amide and neutral amine ligands. In addition, the geometry at N2 and the presence of a peak in the Fourier difference map in the expected position for an amine H atom lead to the assignment of N2 as a neutral dimethylamine ligand.
The Ti—Cl bond distances are heavily influenced by the trans effect, with the longest found for the chloride ligand trans to the dimethylamide group (Table 1). However, the fact that this is the `bridging' Ti—Cl2i [symmetry code: (i) 1 - x, 1 - y, 1 - z] bond also has a strong influence on the bond length. This is confirmed by looking at the Ti—Cl bonds which are trans to each other: the Ti—Cl2 bond is longer than the Ti—Cl3 bond. This effect can only be a result of atom Cl2 being involved in bridging between the two Ti centres, because the immediate environment around the chloride ligands is otherwise identical. The other Ti—Cl distance demonstrates the difference in trans effect between chloride and amine ligands. The Ti—Cl3 distance is the shortest of the four Ti—Cl distances and is a result of atom Cl3 being trans to a bridging chloride ligand. Even though atom Cl1 is trans to a neutral amine ligand instead of an anionic chloride, the stronger trans effect of the amine has the result of lengthening the Ti—Cl1 bond.
These effects were also noted in other structurally characterized examples of [TiCl3LaLn]2 species (La is an anionic amide donor and Ln is a neutral amine donor). Two types of these complexes have been reported in the Cambridge Structural Database (CSD, August 2010 update; Allen, 2002), all of which involve bidentate ligands. Two examples exist of 2-amidopyridine adducts: [Me3SiN(2-py)TiCl3]2 reported by Jones et al. (2003) and [PhN(2-py)TiCl3]2 reported by Talja et al. (2005). Also, two aminosilylamide adducts of TiCl3 have been reported by Buheitel et al. (1996) and Rannabauer et al. (2002), where the donor atoms are linked through an SiX2 group (X = Cl or Me). In each case, the geometry at the metal centre is very similar to that found in (II), with only the nature of the N-containing ligands differing. For example, in each complex, the amide donor is trans to a bridging chloride ligand, resulting in a lengthening of that Ti—Cl bond.
Also present in (II) is an intramolecular hydrogen-bonding interaction between the N2—H2 group of the amine ligand and atom Cl1i (Table 2). Other weaker intermolecular methyl C—H···Cl interactions are also present in the crystal structure (see Table 2) and give rise to a two-dimensional network parallel to the (102) plane, as shown in Fig. 2.
The lack of arsenic in (II) is notable compared with (I), where it was retained. This does rule out (II) as a potential single-source precursor to thin films of TiAs. However, it may prove to be a viable precursor to thin films of TiN, owing to the presence of two N-donor ligands attached to Ti.