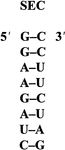issue contents
March 1999 issue
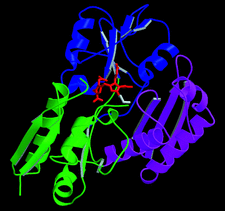
Cover illustration: A ribbon diagram of the catalytically active form of hydroxymethylbilane synthase showing the reduced dipyrrolic cofactor determined de novo by MAD (p. 631).
topical reviews
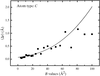
Full-matrix least-squares is used to examine protein precision, both theoretically and by examples, for unrestrained and restrained refinements. Old and new approximate methods are compared critically.
research papers
The crystal structure of distamycin A binding side-by-side to the minor groove of d(GTATATAC)2 has been determined.
NDB reference: distamycin–d(GTATATAC)2, gdh060
The crystal structure of dethiobiotin synthetase has been refined to 0.97 Å resolution. A detailed analysis of the structure is presented.
PDB reference: dethiobiotin synthetase, 1byi
Crystals of a complex of R. miehei aspartic proteinase (RMP) with pepstatin A grew in the space group P212121 and are isomorphous to the native RMP crystals. The structure was solved by molecular replacement.
PDB reference: RMP–pepstatin A complex, 2rmp
The MAD crystal structure of selenomethionine-labelled hydroxymethylbilane synthase, refined at 2.4 Å, is reported with the enzymic cofactor in its active reduced form and spherical density is seen to be consistent with there being a water molecule ideally placed for the final step of the catalytic reaction. As well as showing the effectiveness of the Daresbury SRS Station 9.5 for MAD protein crystallography, this work also establishes the credentials of the CCD for MAD data collection at ESRF Station BM14.
PDB reference: selenomethionine-labelled hydroxymethylbilane synthase, 1ah5
Diffraction data from tetragonal lysozyme crystals grown in microgravity show very small values for mosaic spread (although comparable to those for good quality crystals grown on earth) as well as heterogeneity and anisotropy of the mosaic spread.
An experimental set-up for in-situ diffraction experiments during protein crystal growth has been designed and used to assess the time evolution and spatial distribution of mosaic spread during crystal growth and its relation to growth kinetics.
A numerical model of the equilibration of a hanging-drop experiment is described. The results of the calculations are compared to available experimental data, and the factors that influence the equilibration rate are discussed.
crystallization papers
The acceptor stem of the U6A suppressor tRNASec carries determinants for the binding of the specific elongation factor SELB. The eight-base-pair RNA duplex was crystallized, and X-ray diffraction data were recorded.
Perchloric acid soluble protein – a translational inhibitor – has been crystallized in space group P213. Native diffraction data were collected to 1.8 Å resolution and data for platinum and xenon derivatives to 2.9 and 2.8 Å, respectively.
Human muscle creatine kinase has been crystallized. The crystals are in space group 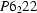 or
or 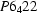 with
with 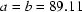 and
and 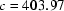 Å and two subunits in the asymmetric unit.
Å and two subunits in the asymmetric unit.
Crystals of both the effector and regulatory domains of Spo0A from Bacillus stearothermophilus have been grown.
A nucleotide-diphospho-sugar transferase involved in bacterial spore synthesis has been cloned, overexpressed and crystallized.
Plastocyanin from Synechococcus sp. PCC7942 has been crystallized in two forms. Data have been obtained using Cu Kα and synchrotron radiation and molecular-replacement calculations performed.
ADP-L-glycero-D-mannoheptose 6-epimerase is a lipopolysaccharide (LPS) core biosynthetic enzyme that catalyzes the interconversion of ADP-D-glycero-D-mannoheptose and ADP-L-glycero-D-mannoheptose. Three different crystal forms have been obtained one suitable for X-ray analysis.
The Bchl protein is one of three subunits of the magnesium chelatase, the enzyme which catalyses the first committed step in chlorophyll and bacteriochlorophyll biosynthesis. Crystals of heterologously expressed R. capsulatus Bchl belong to space group P63 with unit-cell dimensions a = b = 90.6, c = 84.1 Å and diffracted to 2.1 Å resolution.
A novel, highly thermostable phytase from B. amyloliquefaciens has been crystallized. Crystals diffracted to higher than 2.0 Å resolution.
N-carbamyl-D-amino-acid amidohydrolase from A. radiobacter has been expressed in E. coli JM109 and crystallized by vapour diffusion. Crystals diffract to 2.8 Å and are in space group P21 with cell dimensions a = 69.8, b = 67.9 and c = 137.8 Å and β = 96.4°.
Tryparedoxin I from C. fasciculata has been crystallized from PEG 4000 at pH 8.7. The crystals belong to the orthorhombic space group P212121 and diffract to at least 1.7 Å.
DmpA, an L-aminopeptidase from Ochrobactrum anthropi belonging to the Ntn hydrolase family, has been crystallized. Initial screening for heavy-atom derivatives has led to a well substituted Hg derivative and the structure determination by the MIR method is well under way.
Crystallization and preliminary X-ray analysis of the pyrrolidone carboxyl peptidase from the hyperthermophilic archaeon T. litoralis.
A DNA excision repair enzyme, UvrB, from T. thermophilus HB8 has been crystallized. The crystals belong to space group P3121 or P3221 and diffracted X-rays to better than 2.9 Å resolution.
The synthesis of rhamnose is a potential therapeutic target in many bacteria. The preliminary structural study on RmlC, a key enzyme in the synthesis, is described.
short communications
Crystals suitable for structural investigation have been prepared from Δ10Sak, a fully functional staphylokinase derivative which lacks the first ten amino acids.
A complex of the C-terminal domain of B. stearothermophilus initiation factor 2 with fMet-tRNAfMet has been crystallized. Raman spectroscopy proves the presence of both IF2-C and initiator tRNA in the crystals.
Recombinant-derived nucleocapsid of human hepatitis B virus formed a mixture of T = 3 and T = 4 icosahedral particles consisting of 90 and 120 dimers, respectively. The capsids were resolved by sucrose density centrifugation and crystallized; crystals of the T = 4 particles diffracted to 4 Å resolution and the T = 3 particles to 8 Å resolution.
Crystals of aldose reductase, and enzyme involved in diabetes complications, diffract to 1.1 Å resolution at 103 K. Seleno-substituted crystals are also reported.


 journal menu
journal menu