issue contents
December 2004 issue
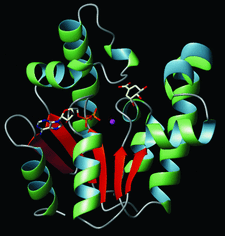
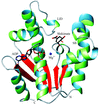
Cover illustration: Crystal structure of shikimate kinase from Mycobacterium tuberculosis (MtSK) complexed with MgADP and shikimic acid (shikimate) has been determined at 2.3 Å resolution, clearly revealing the amino-acid residues involved in shikimate binding. This is the first three-dimensional structure of SK complexed with shikimate. The LID domain (residues 112-124) and shikimate binding domain (SB) (residues 33-61) are responsible for large conformational changes during catalysis (p. 2310).
research papers
The crystal structure of a ribosome-inactivating protein from Himalayan V. album has been determined at 3.8 Å resolution. The structure has revealed unique structural features of the functionally important sites, including the active site, sugar-binding site, antigenic epitopes and ribosome-recognition site.
PDB reference: HmRip, 1pc8, r1pc8sf
The crystal structure of photoactive yellow protein E46Q mutant was determined at 1.2 Å resolution, revealing that the hydrogen bond between p-coumaric acid and Glx46 is lengthened by the mutation. The alkali shift in the pKa, the red shift in the absorption maximum and the rapid photocycle of E46Q can be explained by the weakened hydrogen bond.
PDB reference: photoactive yellow protein E46Q mutant, 1ugu, r1ugusf
The crystal structure of shikimate kinase from Mycobacterium tuberculosis (MtSK) complexed with MgADP and shikimic acid (shikimate) has been determined at 2.3 Å resolution, clearly revealing the amino-acid residues involved in shikimate binding. This is the first three-dimensional structure of shikimate kinase complexed with shikimate.
PDB reference: shikimate kinase–MgADP–shikimate complex, 1we2, r1we2sf
Enzyme structural differences between an alternative substrate and an inhibitor bound at the phosphate site have established the features controlling the oxyanion-specificity of aspartate-β-semialdehyde dehydrogenase.
crystallization papers
NO38 may function as a histone chaperone in Xenopus nucleoli. The conserved N-terminal domain of NO38 has been crystallized as a thermostable pentamer and as a decamer.
TATA box-binding protein (TBP) from M. jannaschii has been crystallized by the hanging-drop vapour-diffusion method. A diffraction data set was collected to 1.9 Å resolution using synchrotron radiation. A molecular-replacement solution was found using the structure of TBP from S. acidocaldarius as a model.
Ferredoxin-NADP(H) reductase from R. capsulatus has been crystallized by the hanging-drop vapour-diffusion method. The addition of a specific detergent is crucial to stabilize disordered regions in the crystal.
The coiled-coil domain of dystrophia myotonica kinase has been cloned, overexpressed, purified and crystallized. Phasing information has been obtained by molecular replacement from models lacking sequence similarity and experimentally by MAD from an SeMet-derivative.
CarBaBb, the class III extradiol dioxygenase involved in carbazole degradation by P. resinovorans strain CA10, has been crystallized and diffraction data were collected to 1.9 Å resolution.
Open  access
access
 access
accessA preliminary crystallographic analysis of the crotonase homologue hydroxycinnamoyl-coenzyme A hydratase-lyase (HCHL) is presented.
The crystallization of the Kelch domain of human Keap1 protein by hanging-drop vapor diffusion is reported in space group P6522.
In order to determine the three-dimensional structure of this protein and to elucidate the underlying molecular mechanisms, the MOMP from C. jejuni strain 85H has been purified and crystallized by vapour diffusion.
A recombinant form of K. pneumoniae maltohexaose-producing α-amylase was overexpressed in E. coli, purified to homogeneity and crystallized by the microbatch technique using polyethylene glycol. The crystal diffracted to better than 2.5 Å resolution at 95 K.
An active form of parasporin-2, a B. thuringiensis crystal protein toxic to particular human cells, has been crystallized. Native data have been collected to 2.75 Å resolution.
The binding protein NgcE of the ABC transporter for N-acetylglucosamine uptake in S. olivaceoviridis was overexpressed and purified. Plate-like crystals were obtained that belonged to either space group P21212 or P212121 and diffracted to 2.2 or 1.8 Å resolution, respectively.
The glutaminyl-tRNA synthetase from D. radiodurans possesses an atypical C-terminal domain the function of which remains unknown. Orthorhombic crystals diffracting to 2.3 Å resolution were obtained from overexpressed protein and characterized using synchrotron radiation. The data allowed solution of the structure by molecular replacement.
A novel 31 kDa segment of the neurofibromatosis type 1 protein has been overexpressed in E. coli, purified and crystallized. Various crystal forms were obtained with a maximum resolution of 2.3 Å.
The bacteriophage φ12 packaging, factor P7 was crystallized, in both full-length and C-terminus truncated forms, P3221, a = 75.7, b = 75.7, c = 45.2 Å, α = 90, β = 90, γ = 120°. Cross-linking indicated dimers of P7 and the need of the N-terminus for dimerization.
The double-zinc aminopeptidase from B. subtilis (BSAP) has been crystallized in a hexagonal space group. Complete zinc MAD data at 2.5 Å resolution and a full native data at 2.2 Å resolution were collected.
Crystals of the fusion core of SARS-CoV spike protein have been grown at 291 K using PEG 4000 as precipitant. The diffraction pattern of the crystal extends to 2.8 Å resolution at 100 K in-house. The crystals have unit-cell parameters a = 121.2, b = 66.3, c = 70.0 Å, α = γ = 90, β = 107.4° and belong to space group C2.
The unliganded form of human growth hormone receptor has been expressed, purified and crystallized by the hanging-drop vapour-diffusion method. The crystals belong to space group C2221 and diffract to 2.5 Å.
Dissimilatory nitrite reductase (CuNIR) isolated from H. denitrificans A3151 exhibits a similar structure to a complex of known green CuNIR and pseudoazurin (or azurin). Crystallographic studies of this enzyme and a mutated form, C260A, which lacks a type I copper ion in the CuNIR-like domain are reported.
The human coactosin-like protein (CLP) has been crystallized by the hanging-drop vapour-diffusion method. Diffraction data have been collected to 1.9 Å resolution.
YciF protein from E. coli was overexpressed and purified. Crystals of YciF in space group R32 diffracted to a resolution of 2.25 Å.
A 9 kDa non-specific lipid-transfer protein (nsLTP) from mung bean (Phaseolus mungo) seeds, displaying antifungal activity, antibacterial activity and lipid-transfer activity, was crystallized at 297 K using ammonium sulfate as a precipitant by means of the hanging-drop vapour-diffusion method.
Single crystals of P. furiosus LamA have been obtained by the hanging-drop vapour-diffusion method using 2-methyl-2,4-pentanediol as a precipitant agent. A complete data set has been collected under cryocooling at a synchrotron source.
The pyridoxal-5′-phosphate-dependent arginine decarboxylase from Y. pestis was crystallized. Crystals diffract to 3.0 Å resolution and belong to the monoclinic space group P21, with unit-cell parameters a = 116.5, b = 78.3, c = 147.3 Å, β = 91.7°. Light scattering indicates ADC to be a dimer of 178 kDa in solution; in the crystalline state the data is consistent with two dimers in the asymmetric unit.
short communications
The direct-methods Shake-and-Bake program SnB was used to obtain the substructure solution from a mutant of E. coli EmrE using data with limited anomalous difference resolution of 5.5 Å.
Open  access
access
 access
accessThe crystal structure of oxalate decarboxylase was solved by manganese SAD using a 2 Å resolution data set collected on a home X-ray source.


 journal menu
journal menu