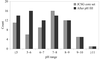issue contents
October 2005 issue
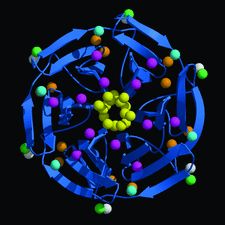
Cover illustration: A ribbon diagram of the Kelch domain of human Keap1 showing the conserved water molecules found in each blade of the ![[beta]](/logos/entities/beta_rmgif.gif) -propeller (p. 1335).
-propeller (p. 1335).
research papers
The crystal structure of the human transthyretin–tetraiodothyroacetic acid complex was determined. The ligand is in an unusual position, penetrating the dimer–dimer interface and interacting with three TTR monomers simultaneously.
PDB reference: transthyretin–T4Ac complex, 1z7j, r1z7jsf
The ternary complex structures of C. neoformans TS and human TS are highly conserved. A 1,8-naphthalein derivative that selectively inhibits C. neoformans and E. coli TSs over human TS binds to an open conformation of E. coli TS. Disorder of the inhibitor-binding site in the human apoenzyme may explain the inhibitor specificity.
Use of 1.35 Å SAD data for de novo structure determination of the Kelch domain and for refinement at atomic resolution is described.
PDB reference: Kelch domain of Keap1, 1zgk, r1zgksf
The crystal structure of a hypothetical protein (MPN555) from M. pneumoniae revealed a trilobal molecule with at least one central binding pocket or channel. The molecule has structural homology with two bacterial chaperone proteins, which suggests a similar chaperone function for MPN555.
PDB reference: MPN555, 1zxj, r1zxjsf
The high-resolution X-ray crystal structures of two anticancer minor-groove-binding quinolinium quaternary salts complexed with the dodecamer d(CGCGAATTCGCG)2 are reported.
Open  access
access
 access
accessUse of the medial axis for automated crystallographic model building is described.
Open  access
access
 access
accessThe work of the Israel Structural Proteomics Center (ISPC) to determine the structures of proteins related to human health in their functional context is described.
Open  access
access
 access
accessThe absolute configuration of haem A, the prosthetic group of cytochrome c oxidase, was determined to be S by analyzing bond angles.
A medium-throughput strategy has been developed for academic structural biology that meets the twin goals of research output and graduate-student development. The platform incorporates Gateway cloning and 96-well methods for solubility screening and crystallization.
Crystallographic analysis of the NNA7 Fab and proposal for the mode of human blood-group recognition
The NNA7 Fab antibody fragment recognizes the human N blood-group antigen, whereas the NNA7-G91S mutant exhibits reduced antigen binding. To provide insight into how these Fab fragments recognize this clinically relevant glycopeptide antigen, the crystal structures of both Fab fragments were solved. The results provide a model for peptide and sugar recognition based upon crystallographic contacts, as well as a bound 2-(N-morpholino)ethanesulfonic acid (MES) molecule trapped in the antigen-combining site.
The crystal structure of fumarase C has been solved in the absence of inhibitors and substrate analogues. This novel crystal structure reports a pH-sensitive shift at the allosteric B site and preservation of the architecture surrounding the conserved active-site water.
PDB reference: fumarase C, 1yfe, r1yfesf
Purified Sesbania mosaic virus coat-protein particles have been crystallized and X-ray crystal structures of their mutant capsids have been determined.
The role of water molecules in the Sesbania mosaic virus structure has been analyzed.
PDB reference: CP-NΔ31, 1x36, r1x36sf
Perdeuterated human aldose reductase neutron diffraction data at 2.2 Å resolution was collected from a 0.15 mm3 crystal. Deuterium atoms were clearly seen in the neutron-scattering density map.
The crystallizability of coagulation factor XI has been improved by surface-residue mutation, generating a quadruple mutant that crystallizes easily in the presence of benzamidine and has facilitated the iterative process of structure-based design of factor XI selective inhibitors.
Open  access
access
 access
accessA strategy for crystallization screening using nanolitre drops and a combination of a sparse-matrix screen (JCSG+) and a novel systematic screen (PACT) is presented and discussed.
essays
Free 

A historical perspective of the steps leading to the current robot technology available for crystal mounting and data collection is presented. The implications for the future of the field are briefly discussed.
book reviews
Open  access
access
 access
access

 journal menu
journal menu