issue contents
July 2007 issue
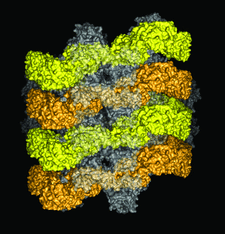
Cover illustration: Bence Jones KWR protein forms a double helical structure, having pseudo sixfold symmetry, about the unique axis of a trigonal crystal. The arrangement resembles the AL-type amyloid fibril model suggested by other immunoglobulin light-chain proteins. Variable domains are shown in yellow and orange, and constant domains in shades of grey (p. 780).
feature articles
Free 

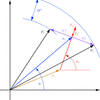
What happens when the signs of anomalous differences or the handedness of substructure are inverted?
The relationships between phases and electron-density maps obtained with correctly or wrongly interpreted anomalous differences or handedness of substructure are discussed.
research papers
It is shown that two major causes of `white-line' variation at the bromine K-absorption edge are, first, the dose-dependent cleavage of C—Br bonds and, second, the crystal orientation versus the beam polarization. The former effect allows monitoring the cleavage of anomalously scattering atoms during data collection, which is important for the RIP method.
The crystal structures of dimeric Rab27b–GDP in three different space groups exhibit an atypical domain swapping of the switch and interswitch regions.
Bence Jones KWR protein, an immunoglobulin light-chain dimer, was crystallized under a variety of conditions after 40 y storage. Structures were obtained with packing arrangements consistent with biochemical and biophysical observations suggestive of an AL-type amyloid-fibril structure. Obtaining molecular-replacement solutions for these crystals proved to be a crystallographic challenge, probably owing to intradomain flexibility and the presence of disordered and proteolytically modified regions.
The application of dual-space fragment extension in the absence of SAD/SIR information makes the technique a general purpose tool for model completion, enabling the combination of direct-method phase refinement with the molecular-replacement technique.
The structure and stability of consensus TPR proteins with eight and 20 repeats and their unusual crystallographic packing resulting from intramolecular symmetry are presented.
Open  access
access
 access
accessInsertion of a dangling 5′-uracil and incorporation of synthetic linkers at the domain interface of a minimal hairpin ribozyme have been investigated as means of favorably influencing crystal packing. These modifications lead to changes in the ribozyme's structural elements that mimic packing within a natural four-way helical junction, thereby providing an example of how knowledge-based design can be used to enhance the diffraction properties of a tertiarily folded RNA.
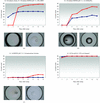
Crystallization of lysozyme solutions reveals that even this robust protein shows very a significant variation in the reproducibility of crystal formation.
The crystal structures of thioredoxin reductase from the gastric pathogen H. pylori in complex with NADP+ have been determined for the oxidized and reduced form of the enzyme at 1.7 and 1.45 Å resolution, respectively.


 journal menu
journal menu