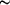 30° rotation about an axis defined by the Hf atom and the ipso-C atom of the benzyl ligand.
30° rotation about an axis defined by the Hf atom and the ipso-C atom of the benzyl ligand.Supporting information
Crystallographic Information File (CIF) https://doi.org/10.1107/S010827010000888X/qa0315sup1.cif | |
Structure factor file (CIF format) https://doi.org/10.1107/S010827010000888X/qa0315Isup2.hkl |
CCDC reference: 150371
A solution of PhCH2MgCl (10.2 ml, 2.0 M in tetrahydrofuran, 20.4 mmol) was added to a slurry of Cp*HfCl3 (2.60 g, 6.19 mmol) in Et2O (30 ml) at 195 K under nitrogen. The reaction mixture was warmed to room temperature and stirred for 1 h, resulting in a pale-yellow slurry. The solvent was removed under vacuum and the resultant solid was extracted with hexane (3 x 40 ml), giving a yellow filtrate. The filtrate was evaporated under vacuum to give a yellow solid which was further purified by recrystallization from toluene/hexane (1.34 g, 36.3%). Crystals suitable for X-ray diffraction were grown from a saturated toluene/hexane solution cooled to 238 K. Analysis calculated for C31H36Hf: C 64.42, H 6.18; found: C 64.50, H 5.99%. 1H NMR (C6D6, 298 K) δ 7.14 (t, J = 7.6 Hz, 6H, meta), 6.91 (t, J = 7.6 Hz, 3H, para), 6.66 (d, J = 7.2 Hz, 6H, ortho), 1.7 (s, 15H, Cp*), 1.68 (s, 6H, CH2). The low temperature 1H NMR spectrum (toluene-d8, 203 K) is essentially unchanged from that at ambient temperature. 13C{H} NMR (C6D6, 298 K): δ 145.9 (ipso-Ph), 128.8, 128.3, 122.9, 119.3 (C5Me5), 83.8 (t, JCH = 121.4 Hz, from gated-{1H} spectrum, HfCH2), 11.7 (C5Me5).
One benzyl ligand (designated with C2** labels) was found to be disordered with two orientations; one is related to the other by a rotation of ~30° about an axis approximated by the Hf—(C22A/C22B average) direction. The occupancies refined to 0.44 (3) for the C2*A site and 0.56 (3) for the C2*B site. Each ligand was refined with individual anisotropic displacement parameters subject to the following restraints: (i) the ring C—C bonds were restrained to 1.39 (1) Å; (ii) the C22A and C22B atoms were assigned the same thermal displacement due to their close proximity (0.138 Å); (iii) each partial benzyl group was restrained to be flat (s.u. = 0.1); (iv) the rigid-bond restraint (s.u. = 0.005) was applied to the displacement parameters of each partially occupied benzyl group; (v) displacement parameters of atoms between the two sites were restrained to be similar if within 0.6 Å (s.u. = 0.05); (iv)) the two sites were restrained to have the same conformation and bonds on opposite sides of the phenyl rings were restrained to be the same (default s.u.'s). H atoms were included with the riding model using program defaults.
Data collection: CAD-4 Operations Manual (Enraf-Nonius, 1977); cell refinement: CAD-4 Operations Manual; data reduction: MolEN (Fair, 1990); program(s) used to solve structure: SHELXTL (Sheldrick, 1995); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
| [Hf(C7H7)3(C10H15)] | Z = 2 |
| Mr = 587.09 | F(000) = 588 |
| Triclinic, P1 | Dx = 1.487 Mg m−3 |
| a = 10.359 (1) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 12.693 (2) Å | Cell parameters from 40 reflections |
| c = 10.102 (7) Å | θ = 6.5–10.2° |
| α = 97.49 (3)° | µ = 3.99 mm−1 |
| β = 91.42 (2)° | T = 293 K |
| γ = 94.96 (1)° | Prism, yellow |
| V = 1311.1 (9) Å3 | 0.56 × 0.42 × 0.40 mm |
| Enraf-Nonius CAD-4 diffractometer | 3907 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.017 |
| Graphite monochromator | θmax = 25.0°, θmin = 1.5° |
| θ–2θ scans | h = −12→12 |
| Absorption correction: ψ scans (MolEN; Fair, 1990) | k = −15→15 |
| Tmin = 0.154, Tmax = 0.202 | l = −11→11 |
| 8828 measured reflections | 4 standard reflections every 60 min |
| 4414 independent reflections | intensity decay: <2% |
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.029 | H-atom parameters constrained |
| wR(F2) = 0.079 | Calculated w = 1/[σ2(Fo2) + (0.0428P)2 + 1.4361P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = −0.031 |
| 4414 reflections | Δρmax = 0.83 e Å−3 |
| 353 parameters | Δρmin = −1.01 e Å−3 |
| 105 restraints | Extinction correction: SHELXTL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0117 (11) |
| [Hf(C7H7)3(C10H15)] | γ = 94.96 (1)° |
| Mr = 587.09 | V = 1311.1 (9) Å3 |
| Triclinic, P1 | Z = 2 |
| a = 10.359 (1) Å | Mo Kα radiation |
| b = 12.693 (2) Å | µ = 3.99 mm−1 |
| c = 10.102 (7) Å | T = 293 K |
| α = 97.49 (3)° | 0.56 × 0.42 × 0.40 mm |
| β = 91.42 (2)° |
| Enraf-Nonius CAD-4 diffractometer | 3907 reflections with I > 2σ(I) |
| Absorption correction: ψ scans (MolEN; Fair, 1990) | Rint = 0.017 |
| Tmin = 0.154, Tmax = 0.202 | 4 standard reflections every 60 min |
| 8828 measured reflections | intensity decay: <2% |
| 4414 independent reflections |
| R[F2 > 2σ(F2)] = 0.029 | 105 restraints |
| wR(F2) = 0.079 | H-atom parameters constrained |
| S = 1.07 | Δρmax = 0.83 e Å−3 |
| 4414 reflections | Δρmin = −1.01 e Å−3 |
| 353 parameters |
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Refinement. Refinement on F2 for ALL reflections except for 0 with very negative F2 or flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Hf1 | 0.20408 (2) | 0.29708 (2) | 0.69427 (2) | 0.06052 (13) | |
| C1 | 0.1191 (5) | 0.4744 (4) | 0.6845 (6) | 0.0725 (13) | |
| C2 | 0.0087 (5) | 0.3998 (5) | 0.6832 (6) | 0.0729 (14) | |
| C3 | 0.0071 (5) | 0.3289 (4) | 0.5626 (5) | 0.0664 (12) | |
| C4 | 0.1164 (5) | 0.3602 (4) | 0.4902 (5) | 0.0648 (12) | |
| C5 | 0.1841 (5) | 0.4502 (4) | 0.5651 (5) | 0.0669 (12) | |
| C6 | 0.1560 (7) | 0.5677 (5) | 0.7906 (7) | 0.100 (2) | |
| H6A | 0.0925 | 0.5699 | 0.8584 | 0.150* | |
| H6B | 0.1596 | 0.6327 | 0.7512 | 0.150* | |
| H6C | 0.2394 | 0.5601 | 0.8300 | 0.150* | |
| C7 | −0.0962 (6) | 0.4022 (7) | 0.7840 (7) | 0.103 (2) | |
| H7A | −0.1636 | 0.4429 | 0.7563 | 0.154* | |
| H7B | −0.0602 | 0.4347 | 0.8697 | 0.154* | |
| H7C | −0.1317 | 0.3307 | 0.7903 | 0.154* | |
| C8 | −0.0981 (5) | 0.2427 (5) | 0.5147 (7) | 0.090 (2) | |
| H8A | −0.0632 | 0.1887 | 0.4541 | 0.135* | |
| H8B | −0.1661 | 0.2726 | 0.4698 | 0.135* | |
| H8C | −0.1323 | 0.2117 | 0.5897 | 0.135* | |
| C9 | 0.1513 (6) | 0.3094 (5) | 0.3557 (6) | 0.085 (2) | |
| H9A | 0.1302 | 0.3538 | 0.2897 | 0.127* | |
| H9B | 0.1035 | 0.2407 | 0.3352 | 0.127* | |
| H9C | 0.2425 | 0.3011 | 0.3558 | 0.127* | |
| C10 | 0.2975 (6) | 0.5171 (5) | 0.5177 (7) | 0.088 (2) | |
| H10A | 0.3499 | 0.5514 | 0.5934 | 0.132* | |
| H10B | 0.2657 | 0.5702 | 0.4689 | 0.132* | |
| H10C | 0.3487 | 0.4721 | 0.4607 | 0.132* | |
| C11 | 0.1776 (5) | 0.1280 (4) | 0.5943 (6) | 0.0776 (14) | |
| H11A | 0.1021 | 0.1195 | 0.5338 | 0.093* | |
| H11B | 0.2525 | 0.1132 | 0.5417 | 0.093* | |
| C12 | 0.1608 (5) | 0.0502 (4) | 0.6903 (6) | 0.0666 (12) | |
| C13 | 0.0384 (5) | 0.0189 (5) | 0.7356 (7) | 0.083 (2) | |
| H13 | −0.0341 | 0.0473 | 0.7035 | 0.100* | |
| C14 | 0.0231 (8) | −0.0519 (6) | 0.8251 (8) | 0.108 (2) | |
| H14 | −0.0593 | −0.0706 | 0.8537 | 0.129* | |
| C15 | 0.1283 (10) | −0.0964 (6) | 0.8741 (8) | 0.109 (2) | |
| H15 | 0.1177 | −0.1455 | 0.9345 | 0.130* | |
| C16 | 0.2495 (8) | −0.0662 (6) | 0.8313 (7) | 0.099 (2) | |
| H16 | 0.3216 | −0.0951 | 0.8634 | 0.119* | |
| C17 | 0.2648 (5) | 0.0059 (5) | 0.7422 (6) | 0.0763 (14) | |
| H17 | 0.3478 | 0.0257 | 0.7158 | 0.092* | |
| C21A | 0.411 (2) | 0.331 (2) | 0.646 (3) | 0.068 (5) | 0.44 (3) |
| H21A | 0.4156 | 0.3323 | 0.5502 | 0.081* | 0.44 (3) |
| H21B | 0.4417 | 0.4014 | 0.6896 | 0.081* | 0.44 (3) |
| C22A | 0.502 (4) | 0.254 (3) | 0.685 (2) | 0.0653 (13) | 0.44 (3) |
| C23A | 0.537 (4) | 0.170 (2) | 0.594 (3) | 0.083 (6) | 0.44 (3) |
| H23A | 0.5043 | 0.1626 | 0.5065 | 0.100* | 0.44 (3) |
| C24A | 0.619 (4) | 0.098 (2) | 0.631 (4) | 0.101 (8) | 0.44 (3) |
| H24A | 0.6424 | 0.0431 | 0.5677 | 0.121* | 0.44 (3) |
| C25A | 0.666 (3) | 0.106 (2) | 0.761 (4) | 0.108 (9) | 0.44 (3) |
| H25A | 0.7175 | 0.0556 | 0.7874 | 0.129* | 0.44 (3) |
| C26A | 0.635 (2) | 0.190 (3) | 0.852 (3) | 0.103 (8) | 0.44 (3) |
| H26A | 0.6691 | 0.1980 | 0.9391 | 0.123* | 0.44 (3) |
| C27A | 0.554 (2) | 0.262 (2) | 0.813 (2) | 0.077 (5) | 0.44 (3) |
| H27A | 0.5340 | 0.3185 | 0.8758 | 0.092* | 0.44 (3) |
| C21B | 0.422 (2) | 0.3485 (14) | 0.700 (3) | 0.074 (4) | 0.56 (3) |
| H21C | 0.4416 | 0.3850 | 0.6231 | 0.089* | 0.56 (3) |
| H21D | 0.4442 | 0.3983 | 0.7798 | 0.089* | 0.56 (3) |
| C22B | 0.501 (3) | 0.255 (2) | 0.698 (2) | 0.0653 (13) | 0.56 (3) |
| C23B | 0.531 (3) | 0.196 (2) | 0.578 (2) | 0.083 (4) | 0.56 (3) |
| H23B | 0.5017 | 0.2159 | 0.4978 | 0.100* | 0.56 (3) |
| C24B | 0.603 (3) | 0.110 (2) | 0.577 (2) | 0.093 (5) | 0.56 (3) |
| H24B | 0.6223 | 0.0720 | 0.4951 | 0.111* | 0.56 (3) |
| C25B | 0.647 (2) | 0.079 (2) | 0.692 (2) | 0.093 (5) | 0.56 (3) |
| H25B | 0.6942 | 0.0194 | 0.6896 | 0.111* | 0.56 (3) |
| C26B | 0.620 (2) | 0.134 (2) | 0.811 (2) | 0.091 (4) | 0.56 (3) |
| H26B | 0.6518 | 0.1152 | 0.8906 | 0.109* | 0.56 (3) |
| C27B | 0.546 (2) | 0.220 (2) | 0.8118 (14) | 0.072 (3) | 0.56 (3) |
| H27B | 0.5260 | 0.2559 | 0.8939 | 0.086* | 0.56 (3) |
| C31 | 0.1577 (6) | 0.3036 (5) | 0.9147 (5) | 0.081 (2) | |
| H31A | 0.1240 | 0.3715 | 0.9434 | 0.098* | |
| H31B | 0.0891 | 0.2481 | 0.9231 | 0.098* | |
| C32 | 0.2657 (5) | 0.2911 (4) | 1.0063 (5) | 0.0668 (12) | |
| C33 | 0.3469 (6) | 0.3786 (4) | 1.0647 (6) | 0.0756 (14) | |
| H33 | 0.3324 | 0.4464 | 1.0455 | 0.091* | |
| C34 | 0.4478 (7) | 0.3667 (6) | 1.1502 (7) | 0.093 (2) | |
| H34 | 0.5001 | 0.4267 | 1.1886 | 0.111* | |
| C35 | 0.4734 (7) | 0.2686 (6) | 1.1801 (7) | 0.097 (2) | |
| H35 | 0.5419 | 0.2613 | 1.2385 | 0.116* | |
| C36 | 0.3958 (7) | 0.1813 (5) | 1.1222 (7) | 0.091 (2) | |
| H36 | 0.4118 | 0.1137 | 1.1409 | 0.109* | |
| C37 | 0.2946 (6) | 0.1928 (4) | 1.0366 (6) | 0.0757 (14) | |
| H37 | 0.2437 | 0.1321 | 0.9978 | 0.091* |
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Hf1 | 0.05347 (15) | 0.0619 (2) | 0.0662 (2) | 0.00708 (8) | 0.00792 (9) | 0.00636 (9) |
| C1 | 0.071 (3) | 0.070 (3) | 0.075 (3) | 0.018 (2) | −0.002 (3) | −0.002 (2) |
| C2 | 0.060 (3) | 0.084 (3) | 0.079 (4) | 0.023 (2) | 0.015 (3) | 0.011 (3) |
| C3 | 0.051 (2) | 0.078 (3) | 0.070 (3) | 0.011 (2) | −0.004 (2) | 0.010 (2) |
| C4 | 0.064 (3) | 0.069 (3) | 0.062 (3) | 0.012 (2) | 0.001 (2) | 0.008 (2) |
| C5 | 0.064 (3) | 0.063 (3) | 0.075 (3) | 0.007 (2) | 0.002 (2) | 0.013 (2) |
| C6 | 0.111 (5) | 0.080 (4) | 0.104 (5) | 0.025 (4) | 0.000 (4) | −0.013 (3) |
| C7 | 0.070 (4) | 0.142 (6) | 0.103 (5) | 0.041 (4) | 0.029 (3) | 0.018 (4) |
| C8 | 0.058 (3) | 0.093 (4) | 0.116 (5) | 0.001 (3) | −0.008 (3) | 0.010 (4) |
| C9 | 0.092 (4) | 0.101 (4) | 0.061 (3) | 0.013 (3) | 0.006 (3) | 0.004 (3) |
| C10 | 0.081 (4) | 0.076 (4) | 0.110 (5) | −0.003 (3) | 0.005 (3) | 0.031 (3) |
| C11 | 0.067 (3) | 0.068 (3) | 0.096 (4) | 0.008 (2) | 0.007 (3) | 0.003 (3) |
| C12 | 0.060 (3) | 0.056 (3) | 0.080 (3) | 0.003 (2) | 0.008 (2) | −0.008 (2) |
| C13 | 0.062 (3) | 0.072 (3) | 0.109 (5) | 0.000 (2) | 0.012 (3) | −0.007 (3) |
| C14 | 0.107 (5) | 0.090 (5) | 0.116 (6) | −0.027 (4) | 0.041 (5) | −0.010 (4) |
| C15 | 0.159 (8) | 0.073 (4) | 0.089 (5) | −0.005 (5) | 0.016 (5) | 0.004 (3) |
| C16 | 0.111 (5) | 0.091 (4) | 0.097 (5) | 0.023 (4) | 0.002 (4) | 0.006 (4) |
| C17 | 0.068 (3) | 0.076 (3) | 0.083 (4) | 0.010 (3) | 0.008 (3) | 0.000 (3) |
| C21A | 0.047 (5) | 0.061 (8) | 0.090 (14) | −0.003 (5) | −0.006 (8) | −0.003 (9) |
| C22A | 0.046 (2) | 0.070 (3) | 0.080 (4) | −0.003 (2) | 0.015 (3) | 0.013 (3) |
| C23A | 0.057 (10) | 0.073 (11) | 0.117 (10) | 0.006 (8) | 0.014 (10) | −0.001 (8) |
| C24A | 0.07 (2) | 0.079 (11) | 0.16 (2) | 0.016 (9) | 0.03 (2) | 0.023 (16) |
| C25A | 0.086 (16) | 0.087 (15) | 0.17 (3) | 0.025 (13) | 0.029 (16) | 0.056 (15) |
| C26A | 0.085 (12) | 0.13 (2) | 0.115 (12) | 0.037 (14) | 0.031 (9) | 0.060 (13) |
| C27A | 0.059 (8) | 0.084 (14) | 0.091 (6) | 0.008 (10) | 0.009 (6) | 0.024 (7) |
| C21B | 0.060 (4) | 0.069 (8) | 0.093 (13) | −0.006 (5) | −0.002 (8) | 0.014 (7) |
| C22B | 0.046 (2) | 0.070 (3) | 0.080 (4) | −0.003 (2) | 0.015 (3) | 0.013 (3) |
| C23B | 0.059 (9) | 0.109 (15) | 0.079 (5) | 0.000 (9) | 0.018 (6) | 0.004 (6) |
| C24B | 0.065 (9) | 0.104 (12) | 0.102 (8) | 0.003 (7) | 0.027 (9) | −0.017 (9) |
| C25B | 0.059 (9) | 0.091 (11) | 0.127 (12) | 0.009 (7) | 0.020 (10) | 0.002 (9) |
| C26B | 0.088 (11) | 0.080 (10) | 0.106 (8) | 0.015 (8) | 0.000 (8) | 0.013 (8) |
| C27B | 0.067 (7) | 0.069 (9) | 0.080 (5) | 0.001 (6) | 0.015 (5) | 0.015 (5) |
| C31 | 0.083 (4) | 0.100 (4) | 0.060 (3) | 0.008 (3) | 0.009 (3) | 0.004 (3) |
| C32 | 0.069 (3) | 0.071 (3) | 0.058 (3) | 0.002 (2) | 0.013 (2) | −0.001 (2) |
| C33 | 0.087 (4) | 0.066 (3) | 0.071 (3) | 0.004 (3) | 0.006 (3) | −0.002 (2) |
| C34 | 0.091 (4) | 0.091 (4) | 0.084 (4) | −0.008 (3) | −0.002 (3) | −0.019 (3) |
| C35 | 0.093 (4) | 0.121 (6) | 0.076 (4) | 0.018 (4) | −0.006 (3) | 0.007 (4) |
| C36 | 0.104 (5) | 0.085 (4) | 0.088 (4) | 0.022 (3) | 0.018 (4) | 0.018 (3) |
| C37 | 0.084 (4) | 0.068 (3) | 0.072 (3) | −0.005 (3) | 0.015 (3) | 0.003 (3) |
| Hf1—C11 | 2.240 (5) | C16—C17 | 1.367 (9) |
| Hf1—C21A | 2.23 (3) | C16—H16 | 0.93 |
| Hf1—C21B | 2.29 (2) | C17—H17 | 0.93 |
| Hf1—C31 | 2.282 (6) | C21A—C22A | 1.492 (14) |
| Hf1—C4 | 2.486 (5) | C21A—H21A | 0.97 |
| Hf1—C5 | 2.499 (5) | C21A—H21B | 0.97 |
| Hf1—C3 | 2.499 (5) | C22A—C27A | 1.383 (9) |
| Hf1—C1 | 2.500 (5) | C22A—C23A | 1.387 (8) |
| Hf1—C2 | 2.508 (5) | C23A—C24A | 1.385 (9) |
| C1—C5 | 1.408 (8) | C23A—H23A | 0.93 |
| C1—C2 | 1.419 (8) | C24A—C25A | 1.379 (9) |
| C1—C6 | 1.506 (8) | C24A—H24A | 0.93 |
| C2—C3 | 1.416 (8) | C25A—C26A | 1.376 (9) |
| C2—C7 | 1.507 (7) | C25A—H25A | 0.93 |
| C3—C4 | 1.419 (7) | C26A—C27A | 1.384 (8) |
| C3—C8 | 1.499 (7) | C26A—H26A | 0.93 |
| C4—C5 | 1.407 (7) | C27A—H27A | 0.93 |
| C4—C9 | 1.492 (7) | C21B—C22B | 1.500 (12) |
| C5—C10 | 1.513 (7) | C21B—H21C | 0.97 |
| C6—H6A | 0.96 | C21B—H21D | 0.97 |
| C6—H6B | 0.96 | C22B—C27B | 1.368 (14) |
| C6—H6C | 0.96 | C22B—C23B | 1.395 (13) |
| C7—H7A | 0.96 | C23B—C24B | 1.383 (14) |
| C7—H7B | 0.96 | C23B—H23B | 0.93 |
| C7—H7C | 0.96 | C24B—C25B | 1.36 (2) |
| C8—H8A | 0.96 | C24B—H24B | 0.93 |
| C8—H8B | 0.96 | C25B—C26B | 1.36 (2) |
| C8—H8C | 0.96 | C25B—H25B | 0.93 |
| C9—H9A | 0.96 | C26B—C27B | 1.385 (15) |
| C9—H9B | 0.96 | C26B—H26B | 0.93 |
| C9—H9C | 0.96 | C27B—H27B | 0.93 |
| C10—H10A | 0.96 | C31—C32 | 1.467 (8) |
| C10—H10B | 0.96 | C31—H31A | 0.97 |
| C10—H10C | 0.96 | C31—H31B | 0.97 |
| C11—C12 | 1.475 (8) | C32—C37 | 1.378 (8) |
| C11—H11A | 0.97 | C32—C33 | 1.390 (7) |
| C11—H11B | 0.97 | C33—C34 | 1.371 (9) |
| C12—C17 | 1.380 (8) | C33—H33 | 0.93 |
| C12—C13 | 1.401 (7) | C34—C35 | 1.365 (10) |
| C13—C14 | 1.358 (10) | C34—H34 | 0.93 |
| C13—H13 | 0.93 | C35—C36 | 1.367 (10) |
| C14—C15 | 1.380 (11) | C35—H35 | 0.93 |
| C14—H14 | 0.93 | C36—C37 | 1.372 (9) |
| C15—C16 | 1.377 (11) | C36—H36 | 0.93 |
| C15—H15 | 0.93 | C37—H37 | 0.93 |
| C21A—Hf1—C11 | 97.3 (6) | C12—C11—H11A | 109.0 |
| C21A—Hf1—C31 | 117.2 (7) | Hf1—C11—H11A | 109.0 |
| C11—Hf1—C31 | 110.0 (2) | C12—C11—H11B | 109.0 |
| C11—Hf1—C21B | 106.8 (5) | Hf1—C11—H11B | 109.0 |
| C31—Hf1—C21B | 103.4 (7) | H11A—C11—H11B | 107.8 |
| C21A—Hf1—C4 | 94.6 (6) | C17—C12—C13 | 116.5 (6) |
| C11—Hf1—C4 | 90.5 (2) | C17—C12—C11 | 121.9 (5) |
| C31—Hf1—C4 | 138.3 (2) | C13—C12—C11 | 121.6 (5) |
| C21B—Hf1—C4 | 104.8 (6) | C14—C13—C12 | 121.6 (6) |
| C21A—Hf1—C5 | 80.3 (5) | C14—C13—H13 | 119.2 |
| C11—Hf1—C5 | 121.3 (2) | C12—C13—H13 | 119.2 |
| C31—Hf1—C5 | 123.1 (2) | C13—C14—C15 | 120.9 (7) |
| C21B—Hf1—C5 | 84.6 (5) | C13—C14—H14 | 119.6 |
| C4—Hf1—C5 | 32.8 (2) | C15—C14—H14 | 119.6 |
| C21A—Hf1—C3 | 127.7 (6) | C16—C15—C14 | 118.4 (7) |
| C11—Hf1—C3 | 87.0 (2) | C16—C15—H15 | 120.8 |
| C31—Hf1—C3 | 109.7 (2) | C14—C15—H15 | 120.8 |
| C21B—Hf1—C3 | 137.0 (6) | C17—C16—C15 | 120.7 (7) |
| C4—Hf1—C3 | 33.1 (2) | C17—C16—H16 | 119.7 |
| C5—Hf1—C3 | 54.5 (2) | C15—C16—H16 | 119.7 |
| C21A—Hf1—C1 | 101.6 (5) | C16—C17—C12 | 122.0 (6) |
| C11—Hf1—C1 | 141.0 (2) | C16—C17—H17 | 119.0 |
| C31—Hf1—C1 | 91.2 (2) | C12—C17—H17 | 119.0 |
| C21B—Hf1—C1 | 99.2 (4) | C22A—C21A—Hf1 | 116 (3) |
| C4—Hf1—C1 | 54.5 (2) | C22A—C21A—H21A | 108.3 |
| C5—Hf1—C1 | 32.7 (2) | Hf1—C21A—H21A | 108.3 |
| C3—Hf1—C1 | 54.5 (2) | C22A—C21A—H21B | 108.2 |
| C21A—Hf1—C2 | 133.2 (5) | Hf1—C21A—H21B | 108.3 |
| C11—Hf1—C2 | 115.1 (2) | H21A—C21A—H21B | 107.4 |
| C31—Hf1—C2 | 83.6 (2) | C27A—C22A—C23A | 116.8 (11) |
| C21B—Hf1—C2 | 132.1 (4) | C27A—C22A—C21A | 121.3 (11) |
| C4—Hf1—C2 | 54.6 (2) | C23A—C22A—C21A | 121.8 (12) |
| C5—Hf1—C2 | 54.4 (2) | C24A—C23A—C22A | 121.9 (12) |
| C3—Hf1—C2 | 32.9 (2) | C24A—C23A—H23A | 119.1 |
| C1—Hf1—C2 | 32.9 (2) | C22A—C23A—H23A | 119.0 |
| C5—C1—C2 | 108.1 (5) | C25A—C24A—C23A | 119.9 (14) |
| C5—C1—C6 | 125.6 (6) | C25A—C24A—H24A | 120.1 |
| C2—C1—C6 | 126.1 (6) | C23A—C24A—H24A | 120.1 |
| C5—C1—Hf1 | 73.6 (3) | C26A—C25A—C24A | 119.4 (14) |
| C2—C1—Hf1 | 73.9 (3) | C26A—C25A—H25A | 120.3 |
| C6—C1—Hf1 | 121.4 (4) | C24A—C25A—H25A | 120.3 |
| C3—C2—C1 | 107.7 (5) | C25A—C26A—C27A | 119.9 (13) |
| C3—C2—C7 | 125.6 (6) | C25A—C26A—H26A | 120.0 |
| C1—C2—C7 | 126.4 (6) | C27A—C26A—H26A | 120.1 |
| C3—C2—Hf1 | 73.2 (3) | C22A—C27A—C26A | 122.1 (12) |
| C1—C2—Hf1 | 73.2 (3) | C22A—C27A—H27A | 119.0 |
| C7—C2—Hf1 | 124.3 (4) | C26A—C27A—H27A | 118.9 |
| C2—C3—C4 | 107.8 (5) | C22B—C21B—Hf1 | 111.9 (19) |
| C2—C3—C8 | 125.8 (5) | C22B—C21B—H21C | 109.3 |
| C4—C3—C8 | 126.2 (5) | Hf1—C21B—H21C | 109.2 |
| C2—C3—Hf1 | 73.9 (3) | C22B—C21B—H21D | 109.2 |
| C4—C3—Hf1 | 72.9 (3) | Hf1—C21B—H21D | 109.2 |
| C8—C3—Hf1 | 123.1 (4) | H21C—C21B—H21D | 107.9 |
| C5—C4—C3 | 108.1 (5) | C27B—C22B—C23B | 115.7 (10) |
| C5—C4—C9 | 125.7 (5) | C27B—C22B—C21B | 123.3 (11) |
| C3—C4—C9 | 126.2 (5) | C23B—C22B—C21B | 121.0 (11) |
| C5—C4—Hf1 | 74.1 (3) | C24B—C23B—C22B | 121.1 (11) |
| C3—C4—Hf1 | 74.0 (3) | C24B—C23B—H23B | 119.5 |
| C9—C4—Hf1 | 119.7 (4) | C22B—C23B—H23B | 119.4 |
| C4—C5—C1 | 108.3 (5) | C25B—C24B—C23B | 121.0 (11) |
| C4—C5—C10 | 125.5 (5) | C25B—C24B—H24B | 119.5 |
| C1—C5—C10 | 125.8 (5) | C23B—C24B—H24B | 119.5 |
| C4—C5—Hf1 | 73.1 (3) | C24B—C25B—C26B | 119.5 (12) |
| C1—C5—Hf1 | 73.7 (3) | C24B—C25B—H25B | 120.2 |
| C10—C5—Hf1 | 124.7 (4) | C26B—C25B—H25B | 120.2 |
| C1—C6—H6A | 109.5 | C25B—C26B—C27B | 119.1 (12) |
| C1—C6—H6B | 109.5 | C25B—C26B—H26B | 120.4 |
| H6A—C6—H6B | 109.5 | C27B—C26B—H26B | 120.4 |
| C1—C6—H6C | 109.5 | C22B—C27B—C26B | 123.5 (11) |
| H6A—C6—H6C | 109.5 | C22B—C27B—H27B | 118.3 |
| H6B—C6—H6C | 109.5 | C26B—C27B—H27B | 118.2 |
| C2—C7—H7A | 109.5 | C32—C31—Hf1 | 116.3 (4) |
| C2—C7—H7B | 109.5 | C32—C31—H31A | 108.2 |
| H7A—C7—H7B | 109.5 | Hf1—C31—H31A | 108.2 |
| C2—C7—H7C | 109.5 | C32—C31—H31B | 108.2 |
| H7A—C7—H7C | 109.5 | Hf1—C31—H31B | 108.2 |
| H7B—C7—H7C | 109.5 | H31A—C31—H31B | 107.4 |
| C3—C8—H8A | 109.5 | C37—C32—C33 | 116.4 (5) |
| C3—C8—H8B | 109.5 | C37—C32—C31 | 122.4 (5) |
| H8A—C8—H8B | 109.5 | C33—C32—C31 | 121.2 (5) |
| C3—C8—H8C | 109.5 | C34—C33—C32 | 121.1 (6) |
| H8A—C8—H8C | 109.5 | C34—C33—H33 | 119.5 |
| H8B—C8—H8C | 109.5 | C32—C33—H33 | 119.5 |
| C4—C9—H9A | 109.5 | C35—C34—C33 | 121.4 (6) |
| C4—C9—H9B | 109.5 | C35—C34—H34 | 119.3 |
| H9A—C9—H9B | 109.5 | C33—C34—H34 | 119.3 |
| C4—C9—H9C | 109.5 | C34—C35—C36 | 118.4 (6) |
| H9A—C9—H9C | 109.5 | C34—C35—H35 | 120.8 |
| H9B—C9—H9C | 109.5 | C36—C35—H35 | 120.8 |
| C5—C10—H10A | 109.5 | C35—C36—C37 | 120.5 (6) |
| C5—C10—H10B | 109.5 | C35—C36—H36 | 119.8 |
| H10A—C10—H10B | 109.5 | C37—C36—H36 | 119.8 |
| C5—C10—H10C | 109.5 | C36—C37—C32 | 122.2 (5) |
| H10A—C10—H10C | 109.5 | C36—C37—H37 | 118.9 |
| H10B—C10—H10C | 109.5 | C32—C37—H37 | 118.9 |
| C12—C11—Hf1 | 112.7 (4) | ||
| C21A—Hf1—C1—C5 | 50.5 (8) | C5—Hf1—C4—C9 | 122.4 (6) |
| C11—Hf1—C1—C5 | −66.9 (4) | C3—Hf1—C4—C9 | −122.9 (6) |
| C31—Hf1—C1—C5 | 168.6 (3) | C1—Hf1—C4—C9 | 159.3 (5) |
| C21B—Hf1—C1—C5 | 64.8 (7) | C2—Hf1—C4—C9 | −160.0 (5) |
| C4—Hf1—C1—C5 | −36.9 (3) | C3—C4—C5—C1 | 0.9 (6) |
| C5—Hf1—C1—C5 | 0.000 (2) | C9—C4—C5—C1 | 178.7 (5) |
| C3—Hf1—C1—C5 | −77.9 (3) | Hf1—C4—C5—C1 | −65.8 (4) |
| C2—Hf1—C1—C5 | −115.0 (5) | C3—C4—C5—C10 | −172.4 (5) |
| C21A—Hf1—C1—C2 | 165.5 (8) | C9—C4—C5—C10 | 5.5 (9) |
| C11—Hf1—C1—C2 | 48.2 (5) | Hf1—C4—C5—C10 | 120.9 (5) |
| C31—Hf1—C1—C2 | −76.4 (4) | C3—C4—C5—Hf1 | 66.7 (4) |
| C21B—Hf1—C1—C2 | 179.8 (7) | C9—C4—C5—Hf1 | −115.4 (5) |
| C4—Hf1—C1—C2 | 78.1 (3) | Hf1—C4—C5—Hf1 | 0.0 |
| C5—Hf1—C1—C2 | 115.0 (5) | C2—C1—C5—C4 | −0.9 (6) |
| C3—Hf1—C1—C2 | 37.2 (3) | C6—C1—C5—C4 | −177.3 (5) |
| C2—Hf1—C1—C2 | 0.0 | Hf1—C1—C5—C4 | 65.5 (4) |
| C21A—Hf1—C1—C6 | −71.7 (9) | C2—C1—C5—C10 | 172.4 (5) |
| C11—Hf1—C1—C6 | 171.0 (5) | C6—C1—C5—C10 | −4.0 (9) |
| C31—Hf1—C1—C6 | 46.4 (5) | Hf1—C1—C5—C10 | −121.3 (5) |
| C21B—Hf1—C1—C6 | −57.3 (8) | C2—C1—C5—Hf1 | −66.3 (4) |
| C4—Hf1—C1—C6 | −159.0 (6) | C6—C1—C5—Hf1 | 117.3 (6) |
| C5—Hf1—C1—C6 | −122.1 (6) | Hf1—C1—C5—Hf1 | 0.000 (1) |
| C3—Hf1—C1—C6 | 160.0 (6) | C21A—Hf1—C5—C4 | 114.6 (7) |
| C2—Hf1—C1—C6 | 122.9 (7) | C11—Hf1—C5—C4 | 21.9 (4) |
| C5—C1—C2—C3 | 0.5 (6) | C31—Hf1—C5—C4 | −129.2 (3) |
| C6—C1—C2—C3 | 176.9 (5) | C21B—Hf1—C5—C4 | 128.3 (7) |
| Hf1—C1—C2—C3 | −65.6 (4) | C4—Hf1—C5—C4 | 0.000 (1) |
| C5—C1—C2—C7 | −173.3 (6) | C3—Hf1—C5—C4 | −37.5 (3) |
| C6—C1—C2—C7 | 3.1 (10) | C1—Hf1—C5—C4 | −115.5 (4) |
| Hf1—C1—C2—C7 | 120.6 (6) | C2—Hf1—C5—C4 | −78.2 (3) |
| C5—C1—C2—Hf1 | 66.1 (4) | C21A—Hf1—C5—C1 | −130.0 (8) |
| C6—C1—C2—Hf1 | −117.5 (6) | C11—Hf1—C5—C1 | 137.3 (3) |
| Hf1—C1—C2—Hf1 | 0.0 | C31—Hf1—C5—C1 | −13.7 (4) |
| C21A—Hf1—C2—C3 | 95.3 (10) | C21B—Hf1—C5—C1 | −116.2 (7) |
| C11—Hf1—C2—C3 | −33.8 (4) | C4—Hf1—C5—C1 | 115.5 (4) |
| C31—Hf1—C2—C3 | −142.9 (4) | C3—Hf1—C5—C1 | 77.9 (3) |
| C21B—Hf1—C2—C3 | 114.8 (9) | C1—Hf1—C5—C1 | 0.000 (3) |
| C4—Hf1—C2—C3 | 37.4 (3) | C2—Hf1—C5—C1 | 37.3 (3) |
| C5—Hf1—C2—C3 | 77.9 (3) | C21A—Hf1—C5—C10 | −7.4 (8) |
| C3—Hf1—C2—C3 | 0.000 (2) | C11—Hf1—C5—C10 | −100.0 (5) |
| C1—Hf1—C2—C3 | 115.0 (5) | C31—Hf1—C5—C10 | 108.9 (5) |
| C21A—Hf1—C2—C1 | −19.7 (10) | C21B—Hf1—C5—C10 | 6.4 (7) |
| C11—Hf1—C2—C1 | −148.8 (3) | C4—Hf1—C5—C10 | −121.9 (6) |
| C31—Hf1—C2—C1 | 102.1 (4) | C3—Hf1—C5—C10 | −159.4 (6) |
| C21B—Hf1—C2—C1 | −0.2 (10) | C1—Hf1—C5—C10 | 122.6 (6) |
| C4—Hf1—C2—C1 | −77.6 (3) | C2—Hf1—C5—C10 | 159.9 (6) |
| C5—Hf1—C2—C1 | −37.0 (3) | C21A—Hf1—C11—C12 | 111.2 (7) |
| C3—Hf1—C2—C1 | −115.0 (5) | C31—Hf1—C11—C12 | −11.2 (4) |
| C1—Hf1—C2—C1 | 0.0 | C21B—Hf1—C11—C12 | 100.3 (7) |
| C21A—Hf1—C2—C7 | −142.6 (11) | C4—Hf1—C11—C12 | −154.0 (4) |
| C11—Hf1—C2—C7 | 88.2 (6) | C5—Hf1—C11—C12 | −165.7 (3) |
| C31—Hf1—C2—C7 | −20.9 (6) | C3—Hf1—C11—C12 | −121.1 (4) |
| C21B—Hf1—C2—C7 | −123.2 (10) | C1—Hf1—C11—C12 | −130.1 (4) |
| C4—Hf1—C2—C7 | 159.5 (6) | C2—Hf1—C11—C12 | −103.5 (4) |
| C5—Hf1—C2—C7 | −160.0 (6) | Hf1—C11—C12—C17 | −90.9 (5) |
| C3—Hf1—C2—C7 | 122.1 (7) | Hf1—C11—C12—C13 | 87.8 (5) |
| C1—Hf1—C2—C7 | −122.9 (7) | C17—C12—C13—C14 | −0.5 (8) |
| C1—C2—C3—C4 | 0.0 (6) | C11—C12—C13—C14 | −179.3 (6) |
| C7—C2—C3—C4 | 173.9 (5) | C12—C13—C14—C15 | −0.5 (10) |
| Hf1—C2—C3—C4 | −65.6 (3) | C13—C14—C15—C16 | 0.8 (11) |
| C1—C2—C3—C8 | −174.9 (5) | C14—C15—C16—C17 | −0.2 (11) |
| C7—C2—C3—C8 | −1.0 (9) | C15—C16—C17—C12 | −0.8 (10) |
| Hf1—C2—C3—C8 | 119.5 (5) | C13—C12—C17—C16 | 1.1 (8) |
| C1—C2—C3—Hf1 | 65.6 (4) | C11—C12—C17—C16 | 179.9 (6) |
| C7—C2—C3—Hf1 | −120.5 (6) | C11—Hf1—C21A—C22A | −53.2 (17) |
| Hf1—C2—C3—Hf1 | 0.000 (1) | C31—Hf1—C21A—C22A | 63.7 (18) |
| C21A—Hf1—C3—C2 | −113.4 (8) | C21B—Hf1—C21A—C22A | 80 (3) |
| C11—Hf1—C3—C2 | 149.7 (3) | C4—Hf1—C21A—C22A | −144.3 (16) |
| C31—Hf1—C3—C2 | 39.5 (4) | C5—Hf1—C21A—C22A | −173.9 (17) |
| C21B—Hf1—C3—C2 | −98.6 (8) | C3—Hf1—C21A—C22A | −145.1 (13) |
| C4—Hf1—C3—C2 | −114.9 (5) | C1—Hf1—C21A—C22A | 161.1 (15) |
| C5—Hf1—C3—C2 | −77.7 (3) | C2—Hf1—C21A—C22A | 171.8 (11) |
| C1—Hf1—C3—C2 | −37.2 (3) | Hf1—C21A—C22A—C27A | −83 (4) |
| C2—Hf1—C3—C2 | 0.000 (3) | Hf1—C21A—C22A—C23A | 97 (3) |
| C21A—Hf1—C3—C4 | 1.5 (8) | C27A—C22A—C23A—C24A | 1 (2) |
| C11—Hf1—C3—C4 | −95.4 (3) | C21A—C22A—C23A—C24A | −179 (4) |
| C31—Hf1—C3—C4 | 154.4 (3) | C22A—C23A—C24A—C25A | 2 (2) |
| C21B—Hf1—C3—C4 | 16.3 (8) | C23A—C24A—C25A—C26A | −3 (4) |
| C4—Hf1—C3—C4 | 0.0 | C24A—C25A—C26A—C27A | 3 (4) |
| C5—Hf1—C3—C4 | 37.2 (3) | C23A—C22A—C27A—C26A | −1 (4) |
| C1—Hf1—C3—C4 | 77.7 (3) | C21A—C22A—C27A—C26A | 178 (3) |
| C2—Hf1—C3—C4 | 114.9 (5) | C25A—C26A—C27A—C22A | −0 (4) |
| C21A—Hf1—C3—C8 | 124.0 (9) | C21A—Hf1—C21B—C22B | −76 (3) |
| C11—Hf1—C3—C8 | 27.1 (5) | C11—Hf1—C21B—C22B | −26.2 (13) |
| C31—Hf1—C3—C8 | −83.1 (5) | C31—Hf1—C21B—C22B | 89.8 (11) |
| C21B—Hf1—C3—C8 | 138.8 (9) | C4—Hf1—C21B—C22B | −121.3 (11) |
| C4—Hf1—C3—C8 | 122.5 (6) | C5—Hf1—C21B—C22B | −147.4 (12) |
| C5—Hf1—C3—C8 | 159.7 (5) | C3—Hf1—C21B—C22B | −130.4 (9) |
| C1—Hf1—C3—C8 | −159.8 (5) | C1—Hf1—C21B—C22B | −176.8 (10) |
| C2—Hf1—C3—C8 | −122.6 (6) | C2—Hf1—C21B—C22B | −176.7 (8) |
| C2—C3—C4—C5 | −0.6 (6) | Hf1—C21B—C22B—C27B | −92 (3) |
| C8—C3—C4—C5 | 174.3 (5) | Hf1—C21B—C22B—C23B | 86 (2) |
| Hf1—C3—C4—C5 | −66.8 (4) | C27B—C22B—C23B—C24B | −1.0 (17) |
| C2—C3—C4—C9 | −178.4 (5) | C21B—C22B—C23B—C24B | −179 (3) |
| C8—C3—C4—C9 | −3.5 (9) | C22B—C23B—C24B—C25B | 0 (2) |
| Hf1—C3—C4—C9 | 115.4 (5) | C23B—C24B—C25B—C26B | −1 (4) |
| C2—C3—C4—Hf1 | 66.3 (4) | C24B—C25B—C26B—C27B | 2 (3) |
| C8—C3—C4—Hf1 | −118.9 (5) | C23B—C22B—C27B—C26B | 2 (3) |
| Hf1—C3—C4—Hf1 | 0.0 | C21B—C22B—C27B—C26B | −180 (3) |
| C21A—Hf1—C4—C5 | −64.1 (7) | C25B—C26B—C27B—C22B | −3 (3) |
| C11—Hf1—C4—C5 | −161.5 (3) | C21A—Hf1—C31—C32 | −21.2 (8) |
| C31—Hf1—C4—C5 | 77.1 (4) | C11—Hf1—C31—C32 | 88.5 (5) |
| C21B—Hf1—C4—C5 | −53.9 (6) | C21B—Hf1—C31—C32 | −25.2 (6) |
| C5—Hf1—C4—C5 | 0.000 (2) | C4—Hf1—C31—C32 | −156.6 (4) |
| C3—Hf1—C4—C5 | 114.7 (4) | C5—Hf1—C31—C32 | −117.6 (4) |
| C1—Hf1—C4—C5 | 36.9 (3) | C3—Hf1—C31—C32 | −177.4 (4) |
| C2—Hf1—C4—C5 | 77.6 (3) | C1—Hf1—C31—C32 | −124.9 (5) |
| C21A—Hf1—C4—C3 | −178.8 (7) | C2—Hf1—C31—C32 | −157.0 (5) |
| C11—Hf1—C4—C3 | 83.8 (3) | Hf1—C31—C32—C37 | −89.6 (6) |
| C31—Hf1—C4—C3 | −37.6 (4) | Hf1—C31—C32—C33 | 88.7 (6) |
| C21B—Hf1—C4—C3 | −168.6 (6) | C37—C32—C33—C34 | −1.5 (8) |
| C5—Hf1—C4—C3 | −114.7 (4) | C31—C32—C33—C34 | −179.9 (5) |
| C3—Hf1—C4—C3 | 0.000 (1) | C32—C33—C34—C35 | 0.7 (10) |
| C1—Hf1—C4—C3 | −77.8 (3) | C33—C34—C35—C36 | 0.3 (11) |
| C2—Hf1—C4—C3 | −37.1 (3) | C34—C35—C36—C37 | −0.3 (10) |
| C21A—Hf1—C4—C9 | 58.3 (7) | C35—C36—C37—C32 | −0.6 (9) |
| C11—Hf1—C4—C9 | −39.1 (4) | C33—C32—C37—C36 | 1.5 (8) |
| C31—Hf1—C4—C9 | −160.5 (4) | C31—C32—C37—C36 | 179.9 (5) |
| C21B—Hf1—C4—C9 | 68.5 (6) |
Experimental details
| Crystal data | |
| Chemical formula | [Hf(C7H7)3(C10H15)] |
| Mr | 587.09 |
| Crystal system, space group | Triclinic, P1 |
| Temperature (K) | 293 |
| a, b, c (Å) | 10.359 (1), 12.693 (2), 10.102 (7) |
| α, β, γ (°) | 97.49 (3), 91.42 (2), 94.96 (1) |
| V (Å3) | 1311.1 (9) |
| Z | 2 |
| Radiation type | Mo Kα |
| µ (mm−1) | 3.99 |
| Crystal size (mm) | 0.56 × 0.42 × 0.40 |
| Data collection | |
| Diffractometer | Enraf-Nonius CAD-4 diffractometer |
| Absorption correction | ψ scans (MolEN; Fair, 1990) |
| Tmin, Tmax | 0.154, 0.202 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 8828, 4414, 3907 |
| Rint | 0.017 |
| (sin θ/λ)max (Å−1) | 0.595 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.029, 0.079, 1.07 |
| No. of reflections | 4414 |
| No. of parameters | 353 |
| No. of restraints | 105 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.83, −1.01 |
Computer programs: CAD-4 Operations Manual (Enraf-Nonius, 1977), CAD-4 Operations Manual, MolEN (Fair, 1990), SHELXTL (Sheldrick, 1995), SHELXTL.
| Hf1—C11 | 2.240 (5) | C15—C16 | 1.377 (11) |
| Hf1—C21A | 2.23 (3) | C16—C17 | 1.367 (9) |
| Hf1—C21B | 2.29 (2) | C21A—C22A | 1.492 (14) |
| Hf1—C31 | 2.282 (6) | C22A—C27A | 1.383 (9) |
| Hf1—C4 | 2.486 (5) | C22A—C23A | 1.387 (8) |
| Hf1—C5 | 2.499 (5) | C23A—C24A | 1.385 (9) |
| Hf1—C3 | 2.499 (5) | C24A—C25A | 1.379 (9) |
| Hf1—C1 | 2.500 (5) | C25A—C26A | 1.376 (9) |
| Hf1—C2 | 2.508 (5) | C26A—C27A | 1.384 (8) |
| C1—C5 | 1.408 (8) | C21B—C22B | 1.500 (12) |
| C1—C2 | 1.419 (8) | C22B—C27B | 1.368 (14) |
| C1—C6 | 1.506 (8) | C22B—C23B | 1.395 (13) |
| C2—C3 | 1.416 (8) | C23B—C24B | 1.383 (14) |
| C2—C7 | 1.507 (7) | C24B—C25B | 1.36 (2) |
| C3—C4 | 1.419 (7) | C25B—C26B | 1.36 (2) |
| C3—C8 | 1.499 (7) | C26B—C27B | 1.385 (15) |
| C4—C5 | 1.407 (7) | C31—C32 | 1.467 (8) |
| C4—C9 | 1.492 (7) | C32—C37 | 1.378 (8) |
| C5—C10 | 1.513 (7) | C32—C33 | 1.390 (7) |
| C11—C12 | 1.475 (8) | C33—C34 | 1.371 (9) |
| C12—C17 | 1.380 (8) | C34—C35 | 1.365 (10) |
| C12—C13 | 1.401 (7) | C35—C36 | 1.367 (10) |
| C13—C14 | 1.358 (10) | C36—C37 | 1.372 (9) |
| C14—C15 | 1.380 (11) | ||
| C21A—Hf1—C11 | 97.3 (6) | C16—C17—C12 | 122.0 (6) |
| C21A—Hf1—C31 | 117.2 (7) | C22A—C21A—Hf1 | 116 (3) |
| C11—Hf1—C31 | 110.0 (2) | C27A—C22A—C23A | 116.8 (11) |
| C11—Hf1—C21B | 106.8 (5) | C27A—C22A—C21A | 121.3 (11) |
| C31—Hf1—C21B | 103.4 (7) | C23A—C22A—C21A | 121.8 (12) |
| C5—C1—C2 | 108.1 (5) | C24A—C23A—C22A | 121.9 (12) |
| C5—C1—C6 | 125.6 (6) | C25A—C24A—C23A | 119.9 (14) |
| C2—C1—C6 | 126.1 (6) | C26A—C25A—C24A | 119.4 (14) |
| C3—C2—C1 | 107.7 (5) | C25A—C26A—C27A | 119.9 (13) |
| C3—C2—C7 | 125.6 (6) | C22A—C27A—C26A | 122.1 (12) |
| C1—C2—C7 | 126.4 (6) | C22B—C21B—Hf1 | 111.9 (19) |
| C2—C3—C4 | 107.8 (5) | C27B—C22B—C23B | 115.7 (10) |
| C2—C3—C8 | 125.8 (5) | C27B—C22B—C21B | 123.3 (11) |
| C4—C3—C8 | 126.2 (5) | C23B—C22B—C21B | 121.0 (11) |
| C5—C4—C3 | 108.1 (5) | C24B—C23B—C22B | 121.1 (11) |
| C5—C4—C9 | 125.7 (5) | C25B—C24B—C23B | 121.0 (11) |
| C3—C4—C9 | 126.2 (5) | C24B—C25B—C26B | 119.5 (12) |
| C4—C5—C1 | 108.3 (5) | C25B—C26B—C27B | 119.1 (12) |
| C4—C5—C10 | 125.5 (5) | C22B—C27B—C26B | 123.5 (11) |
| C1—C5—C10 | 125.8 (5) | C32—C31—Hf1 | 116.3 (4) |
| C12—C11—Hf1 | 112.7 (4) | C37—C32—C33 | 116.4 (5) |
| C17—C12—C13 | 116.5 (6) | C37—C32—C31 | 122.4 (5) |
| C17—C12—C11 | 121.9 (5) | C33—C32—C31 | 121.2 (5) |
| C13—C12—C11 | 121.6 (5) | C34—C33—C32 | 121.1 (6) |
| C14—C13—C12 | 121.6 (6) | C35—C34—C33 | 121.4 (6) |
| C13—C14—C15 | 120.9 (7) | C34—C35—C36 | 118.4 (6) |
| C16—C15—C14 | 118.4 (7) | C35—C36—C37 | 120.5 (6) |
| C17—C16—C15 | 120.7 (7) | C36—C37—C32 | 122.2 (5) |

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
The full text of this article is available to subscribers to the journal.
- Information on subscribing
- Sample issue
- Purchase subscription
- Reduced-price subscriptions
- If you have already subscribed, you may need to register

 journal menu
journal menu








![[eta]](/logos/entities/eta_rmgif.gif)




The structures of electronically unsaturated early transition metal benzyl complexes are often distorted by weak M—Ph interactions which lead to reduced M—CH2—Ph angles and short M···Cipso contacts. Such η2– or ηn-benzyl structures were first observed for M(CH2Ph)4 (M = Ti, Zr, Hf) complexes (Davies et al., 1971; Bassi et al., 1971) and have since been observed in a variety of systems, including cationic group 4 metal benzyl compounds (Jordan et al., 1987, 1990; Crowther et al., 1993; Bei et al., 1997).
The title compound, (I), adopts a monomeric three-legged piano-stool structure. The plane formed by the three methylene C atoms (C11, C21A and C21B average, and C31) is parallel to the pentamethylcyclopentadienyl (Cp*) plane (dihedral angle = 0.5°). The Cp* methyl groups are bent slightly away from the Hf atom, with the largest deviation [0.166 (10) Å from the C1–C5 plane] occurring with the C10-methyl group that is eclipsed with the disordered benzyl ligand [C21A—C27A, occupancy = 0.44 (3); C21B–C27B, occupancy = 0.56 (3)]. There are no short Hf···Cipso contacts (no distances less than 3.1 Å), indicating no significant Hf—Ph interaction.