Buy article online - an online subscription or single-article purchase is required to access this article.
The pyrimidine rings in ethyl (
E)-3-[2-amino-4,6-bis(dimethylamino)pyrimidin-5-yl]-2-cyanoacrylate, C
14H
20N
6O
2, (I), and 2-[(2-amino-4,6-di-1-piperidylpyrimidin-5-yl)methylene]malononitrile, C
18H
23N
7, (II), which crystallizes with
Z' = 2 in the
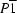
space group, are both nonplanar with boat conformations. The molecules of (I) are linked by a combination of N-H

N and N-H

O hydrogen bonds into chains of edge-fused
R22(8) and
R44(20) rings, while the two independent molecules in (II) are linked by four N-H

N hydrogen bonds into chains of edge-fused
R22(8) and
R22(20) rings. This study illustrates both the readiness with which highly-substituted pyrimidine rings can be distorted from planarity and the significant differences between the supramolecular aggregation in two rather similar compounds.
Supporting information
CCDC references: 682823; 682824
For the synthesis of compound (I), a catalytic quantity of triethylamine (3
drops) together with calcium chloride (15 mg) were added to a solution of
2-amino-4,6-dichloropyrimidine-5-carbaldehyde (1.0 mmol) and ethyl
2-cyanoacetate (1.0 mmol) in ethanol (10 ml), and this mixture was stirred at
ambient temperature for 2 h. The resulting precipitate was collected by
filtration, washed with ethanol, dried and finally recrystallized from ethanol
to give ethyl
(E)-3-(2-amino-4,6-dichloropyrimidin-5-yl)-2-cyanoacrylate, (III), in
85% yield. For the synthesis of compound (II), a catalytic quantity of
triethylamine (3 drops) together with calcium chloride (15 mg) were added to a
solution of 2-amino-4,6-dichloropyrimidine-5-carbaldehyde (1.0 mmol) and
malononitrile (1.0 mmol) in ethanol (10 ml) and this mixture was stirred at
ambient temperature for 2 h. The resulting solid was collected by filtration,
washed with ethanol, dried and finally recrystallized from ethanol to give
2-[(2-amino-4,6-dichloropyrimidin-5-yl)methylene]malononitrile, (IV), in 70%
yield. For the conversion of (II) and (IV) to (I) and (II), respectively, the
intermediate (III) or (IV) (0.4 mmol) was then added to a large excess of the
appropriate amine (0.5 ml) in ethanol (10 ml) and heated under reflux for 1 h.
On cooling to ambient temperature, the products (I) and (II) were precipitated
as yellow crystalline solids, which were collected by filtration, washed with
ethanol and dried at atmospheric pressure to give crystals suitable for
single-crystal X-ray diffraction. For (I): yield 50% and m.p. 503–505 K; for
(II), yield 70% and m.p. 467–468 K. HR–MS: found 337.2016; C18H23N7
requires 337.2015.
Crystals of compounds (I) and (II) are triclinic; for each compound the space
group P-1 was selected and confirmed by the subsequent structure analysis. All
H atoms were located in difference maps and then treated as riding atoms with
distances C—H = 0.95 (alkene), 0.98 (CH3) or 0.99 Å (CH2) and N—H =
0.86 Å, and with Uiso(H) = kUeq(carrier), where k =
1.5 for the methyl groups and 1.2 for all other H atoms. For compound (I), the
proportion of the reflections labelled `observed' was quite low, ca
49%, even at 120 K. Analysis of the refined structure of (II) using
PLATON (Spek, 2003) showed that there were voids within the structure,
centred at approximately (0, 0, 1/2) and accounting for some 13.6% of the
total unit-cell volume. Several significant peaks corresponding to electron
densities up to 2.02 e Å-3 were located within the void space. However,
these peaks could not be reconciled with any plausible molecular species,
possibly because of disorder and/or mobility. Accordingly, the SQUEEZE option
in PLATON was utilized; while this reduced R from 0.104 to
0.0646, the quality of the data set did not permit a meaningful evaluation of
the electron population in the void.
For both compounds, data collection: COLLECT (Hooft, 1999); cell refinement: DIRAX/LSQ (Duisenberg et al., 2000); data reduction: EVALCCD (Duisenberg et al., 2003); program(s) used to solve structure: SIR2004 (Burla et al., 2005); program(s) used to refine structure: OSCAIL (McArdle, 2003) and SHELXL97 (Sheldrick, 2008); molecular graphics: PLATON (Spek, 2003); software used to prepare material for publication: SHELXL97 (Sheldrick, 2008) and PRPKAPPA (Ferguson, 1999).
(I) ethyl
(
E)-3-[2-amino-4,6-bis(dimethylamino)pyrimidin-5-yl]-2-cyanoacrylate
top
Crystal data top
| C14H20N6O2 | Z = 2 |
| Mr = 304.36 | F(000) = 324 |
| Triclinic, P1 | Dx = 1.331 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.5570 (15) Å | Cell parameters from 3468 reflections |
| b = 9.893 (2) Å | θ = 4.1–27.5° |
| c = 9.9739 (18) Å | µ = 0.09 mm−1 |
| α = 87.534 (13)° | T = 120 K |
| β = 77.251 (15)° | Block, colourless |
| γ = 67.431 (14)° | 0.37 × 0.34 × 0.15 mm |
| V = 759.6 (3) Å3 | |
Data collection top
Bruker–Nonius KappaCCD
diffractometer | 3468 independent reflections |
| Radiation source: Bruker–Nonius FR591 rotating anode | 1715 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.085 |
| Detector resolution: 9.091 pixels mm-1 | θmax = 27.5°, θmin = 4.1° |
| ϕ and ω scans | h = −11→11 |
Absorption correction: multi-scan
(SADABS; Sheldrick, 2003) | k = −12→12 |
| Tmin = 0.971, Tmax = 0.986 | l = −12→12 |
| 18938 measured reflections | |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.069 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.236 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.130P)2 + 0.0863P]
where P = (Fo2 + 2Fc2)/3 |
| 3468 reflections | (Δ/σ)max < 0.001 |
| 204 parameters | Δρmax = 0.44 e Å−3 |
| 0 restraints | Δρmin = −0.37 e Å−3 |
Crystal data top
| C14H20N6O2 | γ = 67.431 (14)° |
| Mr = 304.36 | V = 759.6 (3) Å3 |
| Triclinic, P1 | Z = 2 |
| a = 8.5570 (15) Å | Mo Kα radiation |
| b = 9.893 (2) Å | µ = 0.09 mm−1 |
| c = 9.9739 (18) Å | T = 120 K |
| α = 87.534 (13)° | 0.37 × 0.34 × 0.15 mm |
| β = 77.251 (15)° | |
Data collection top
Bruker–Nonius KappaCCD
diffractometer | 3468 independent reflections |
Absorption correction: multi-scan
(SADABS; Sheldrick, 2003) | 1715 reflections with I > 2σ(I) |
| Tmin = 0.971, Tmax = 0.986 | Rint = 0.085 |
| 18938 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.069 | 0 restraints |
| wR(F2) = 0.236 | H-atom parameters constrained |
| S = 1.03 | Δρmax = 0.44 e Å−3 |
| 3468 reflections | Δρmin = −0.37 e Å−3 |
| 204 parameters | |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| O55 | 0.5647 (3) | 0.7337 (2) | −0.1477 (2) | 0.0290 (6) | |
| O56 | 0.3443 (3) | 0.9501 (2) | −0.0864 (2) | 0.0288 (6) | |
| N1 | 0.8254 (3) | 0.5391 (3) | 0.3715 (2) | 0.0235 (6) | |
| N2 | 0.7796 (3) | 0.6729 (3) | 0.5677 (3) | 0.0275 (7) | |
| N3 | 0.5387 (3) | 0.6968 (3) | 0.4920 (2) | 0.0245 (6) | |
| N4 | 0.3022 (3) | 0.7117 (3) | 0.4087 (2) | 0.0230 (6) | |
| N6 | 0.8682 (3) | 0.4237 (3) | 0.1636 (2) | 0.0241 (6) | |
| N54 | 0.2080 (4) | 1.0044 (3) | 0.2559 (3) | 0.0377 (8) | |
| C2 | 0.7122 (4) | 0.6373 (3) | 0.4726 (3) | 0.0241 (7) | |
| C4 | 0.4739 (4) | 0.6763 (3) | 0.3874 (3) | 0.0238 (7) | |
| C5 | 0.5859 (4) | 0.6154 (3) | 0.2558 (3) | 0.0233 (7) | |
| C6 | 0.7613 (4) | 0.5227 (3) | 0.2651 (3) | 0.0216 (7) | |
| C41 | 0.2248 (4) | 0.6563 (4) | 0.3186 (3) | 0.0283 (8) | |
| C42 | 0.1864 (4) | 0.8013 (4) | 0.5305 (3) | 0.0321 (8) | |
| C51 | 0.5569 (4) | 0.6707 (3) | 0.1280 (3) | 0.0219 (7) | |
| C52 | 0.4365 (4) | 0.7968 (3) | 0.0886 (3) | 0.0221 (7) | |
| C53 | 0.3089 (4) | 0.9116 (4) | 0.1809 (3) | 0.0256 (7) | |
| C55 | 0.4578 (4) | 0.8208 (3) | −0.0587 (3) | 0.0227 (7) | |
| C57 | 0.3524 (4) | 0.9805 (4) | −0.2306 (3) | 0.0297 (8) | |
| C58 | 0.1876 (4) | 1.1043 (4) | −0.2409 (3) | 0.0350 (9) | |
| C61 | 1.0537 (4) | 0.3587 (4) | 0.1554 (3) | 0.0280 (8) | |
| C62 | 0.8051 (4) | 0.3512 (4) | 0.0764 (3) | 0.0291 (8) | |
| H2A | 0.8847 | 0.6190 | 0.5716 | 0.033* | |
| H2B | 0.7073 | 0.7204 | 0.6406 | 0.033* | |
| H41A | 0.3116 | 0.5652 | 0.2704 | 0.042* | |
| H41B | 0.1849 | 0.7293 | 0.2512 | 0.042* | |
| H41C | 0.1261 | 0.6372 | 0.3738 | 0.042* | |
| H42A | 0.2065 | 0.8920 | 0.5346 | 0.048* | |
| H42B | 0.2093 | 0.7469 | 0.6131 | 0.048* | |
| H42C | 0.0656 | 0.8253 | 0.5255 | 0.048* | |
| H51 | 0.6365 | 0.6086 | 0.0523 | 0.026* | |
| H57A | 0.3656 | 0.8926 | −0.2832 | 0.036* | |
| H57B | 0.4530 | 1.0074 | −0.2689 | 0.036* | |
| H58A | 0.1746 | 1.1899 | −0.1869 | 0.052* | |
| H58B | 0.0892 | 1.0754 | −0.2052 | 0.052* | |
| H58C | 0.1907 | 1.1289 | −0.3375 | 0.052* | |
| H61A | 1.0811 | 0.2714 | 0.2102 | 0.042* | |
| H61B | 1.0903 | 0.4298 | 0.1912 | 0.042* | |
| H61C | 1.1151 | 0.3309 | 0.0593 | 0.042* | |
| H62A | 0.6794 | 0.3806 | 0.1086 | 0.044* | |
| H62B | 0.8624 | 0.2448 | 0.0806 | 0.044* | |
| H62C | 0.8310 | 0.3795 | −0.0188 | 0.044* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| O55 | 0.0259 (12) | 0.0319 (13) | 0.0211 (12) | −0.0040 (10) | −0.0024 (10) | 0.0013 (10) |
| O56 | 0.0296 (12) | 0.0282 (13) | 0.0224 (12) | −0.0054 (10) | −0.0047 (9) | 0.0031 (10) |
| N1 | 0.0249 (14) | 0.0253 (15) | 0.0189 (14) | −0.0081 (12) | −0.0050 (11) | 0.0005 (12) |
| N2 | 0.0272 (15) | 0.0320 (16) | 0.0214 (14) | −0.0082 (13) | −0.0064 (11) | −0.0033 (12) |
| N3 | 0.0255 (15) | 0.0286 (15) | 0.0187 (14) | −0.0093 (12) | −0.0056 (11) | 0.0020 (12) |
| N4 | 0.0212 (14) | 0.0275 (15) | 0.0186 (13) | −0.0081 (12) | −0.0031 (11) | −0.0003 (11) |
| N6 | 0.0233 (14) | 0.0251 (15) | 0.0209 (14) | −0.0049 (12) | −0.0062 (11) | −0.0029 (12) |
| N54 | 0.0445 (18) | 0.0276 (16) | 0.0329 (17) | −0.0080 (14) | −0.0032 (14) | 0.0030 (14) |
| C2 | 0.0283 (19) | 0.0220 (17) | 0.0194 (16) | −0.0083 (14) | −0.0036 (13) | 0.0051 (13) |
| C4 | 0.0238 (17) | 0.0225 (17) | 0.0227 (17) | −0.0066 (14) | −0.0051 (13) | 0.0014 (14) |
| C5 | 0.0235 (16) | 0.0277 (18) | 0.0190 (16) | −0.0107 (14) | −0.0029 (13) | −0.0015 (13) |
| C6 | 0.0261 (17) | 0.0203 (17) | 0.0188 (16) | −0.0104 (14) | −0.0041 (13) | 0.0054 (13) |
| C41 | 0.0251 (17) | 0.0304 (19) | 0.0277 (18) | −0.0102 (15) | −0.0036 (14) | 0.0016 (15) |
| C42 | 0.0285 (19) | 0.0331 (19) | 0.0255 (18) | −0.0056 (15) | 0.0012 (14) | −0.0019 (15) |
| C51 | 0.0198 (16) | 0.0244 (17) | 0.0212 (16) | −0.0088 (14) | −0.0029 (12) | −0.0014 (14) |
| C52 | 0.0227 (17) | 0.0237 (17) | 0.0185 (16) | −0.0071 (14) | −0.0050 (12) | 0.0010 (13) |
| C53 | 0.0294 (19) | 0.0247 (18) | 0.0223 (17) | −0.0097 (15) | −0.0069 (14) | 0.0060 (15) |
| C55 | 0.0179 (16) | 0.0247 (18) | 0.0259 (18) | −0.0083 (14) | −0.0057 (14) | 0.0024 (15) |
| C57 | 0.0331 (19) | 0.035 (2) | 0.0212 (17) | −0.0116 (16) | −0.0111 (14) | 0.0077 (15) |
| C58 | 0.041 (2) | 0.032 (2) | 0.0305 (19) | −0.0089 (17) | −0.0162 (16) | 0.0092 (16) |
| C61 | 0.0225 (17) | 0.0329 (19) | 0.0256 (18) | −0.0068 (15) | −0.0062 (14) | 0.0016 (15) |
| C62 | 0.0315 (19) | 0.0297 (19) | 0.0265 (18) | −0.0118 (16) | −0.0058 (14) | −0.0042 (15) |
Geometric parameters (Å, º) top
| O55—C55 | 1.212 (3) | C41—H41B | 0.98 |
| O56—C55 | 1.338 (4) | C41—H41C | 0.98 |
| O56—C57 | 1.449 (3) | C42—H42A | 0.98 |
| N1—C6 | 1.340 (4) | C42—H42B | 0.98 |
| N1—C2 | 1.353 (4) | C42—H42C | 0.98 |
| N2—C2 | 1.335 (4) | C51—C52 | 1.387 (4) |
| N2—H2A | 0.8601 | C51—H51 | 0.95 |
| N2—H2B | 0.86 | C52—C53 | 1.423 (4) |
| N3—C4 | 1.340 (4) | C52—C55 | 1.460 (4) |
| N3—C2 | 1.342 (4) | C57—C58 | 1.492 (4) |
| N4—C4 | 1.342 (4) | C57—H57A | 0.99 |
| N4—C41 | 1.459 (4) | C57—H57B | 0.99 |
| N4—C42 | 1.460 (4) | C58—H58A | 0.98 |
| N6—C6 | 1.345 (4) | C58—H58B | 0.98 |
| N6—C61 | 1.451 (4) | C58—H58C | 0.98 |
| N6—C62 | 1.456 (4) | C61—H61A | 0.98 |
| N54—C53 | 1.146 (4) | C61—H61B | 0.98 |
| C4—C5 | 1.440 (4) | C61—H61C | 0.98 |
| C5—C51 | 1.400 (4) | C62—H62A | 0.98 |
| C5—C6 | 1.449 (4) | C62—H62B | 0.98 |
| C41—H41A | 0.98 | C62—H62C | 0.98 |
| | | |
| C55—O56—C57 | 116.3 (2) | H42B—C42—H42C | 109.5 |
| C6—N1—C2 | 115.8 (3) | C52—C51—C5 | 133.6 (3) |
| C2—N2—H2A | 118.2 | C52—C51—H51 | 113.2 |
| C2—N2—H2B | 116.5 | C5—C51—H51 | 113.2 |
| H2A—N2—H2B | 119.7 | C51—C52—C53 | 124.7 (3) |
| C4—N3—C2 | 115.8 (3) | C51—C52—C55 | 116.9 (3) |
| C4—N4—C41 | 122.5 (3) | C53—C52—C55 | 117.9 (3) |
| C4—N4—C42 | 119.9 (3) | N54—C53—C52 | 178.9 (3) |
| C41—N4—C42 | 117.4 (3) | O55—C55—O56 | 122.8 (3) |
| C6—N6—C61 | 120.9 (3) | O55—C55—C52 | 124.5 (3) |
| C6—N6—C62 | 122.5 (3) | O56—C55—C52 | 112.7 (3) |
| C61—N6—C62 | 115.1 (3) | O56—C57—C58 | 107.7 (2) |
| N2—C2—N3 | 116.4 (3) | O56—C57—H57A | 110.2 |
| N2—C2—N1 | 116.5 (3) | C58—C57—H57A | 110.2 |
| N3—C2—N1 | 127.1 (3) | O56—C57—H57B | 110.2 |
| N3—C4—N4 | 118.5 (3) | C58—C57—H57B | 110.2 |
| N3—C4—C5 | 121.0 (3) | H57A—C57—H57B | 108.5 |
| N4—C4—C5 | 120.6 (3) | C57—C58—H58A | 109.5 |
| C51—C5—C4 | 125.4 (3) | C57—C58—H58B | 109.5 |
| C51—C5—C6 | 118.4 (3) | H58A—C58—H58B | 109.5 |
| C4—C5—C6 | 113.1 (3) | C57—C58—H58C | 109.5 |
| N1—C6—N6 | 117.8 (3) | H58A—C58—H58C | 109.5 |
| N1—C6—C5 | 120.8 (3) | H58B—C58—H58C | 109.5 |
| N6—C6—C5 | 121.2 (3) | N6—C61—H61A | 109.5 |
| N4—C41—H41A | 109.5 | N6—C61—H61B | 109.5 |
| N4—C41—H41B | 109.5 | H61A—C61—H61B | 109.5 |
| H41A—C41—H41B | 109.5 | N6—C61—H61C | 109.5 |
| N4—C41—H41C | 109.5 | H61A—C61—H61C | 109.5 |
| H41A—C41—H41C | 109.5 | H61B—C61—H61C | 109.5 |
| H41B—C41—H41C | 109.5 | N6—C62—H62A | 109.5 |
| N4—C42—H42A | 109.5 | N6—C62—H62B | 109.5 |
| N4—C42—H42B | 109.5 | H62A—C62—H62B | 109.5 |
| H42A—C42—H42B | 109.5 | N6—C62—H62C | 109.5 |
| N4—C42—H42C | 109.5 | H62A—C62—H62C | 109.5 |
| H42A—C42—H42C | 109.5 | H62B—C62—H62C | 109.5 |
| | | |
| C4—N3—C2—N2 | 171.5 (3) | C61—N6—C6—C5 | 163.9 (3) |
| C4—N3—C2—N1 | −12.5 (4) | C62—N6—C6—C5 | −30.4 (4) |
| C6—N1—C2—N2 | −169.3 (3) | C51—C5—C6—N1 | 137.2 (3) |
| C6—N1—C2—N3 | 14.7 (5) | C4—C5—C6—N1 | −24.0 (4) |
| C2—N3—C4—N4 | 169.2 (3) | C51—C5—C6—N6 | −38.8 (4) |
| C2—N3—C4—C5 | −9.7 (4) | C4—C5—C6—N6 | 160.1 (3) |
| C41—N4—C4—N3 | −161.8 (3) | C4—C5—C51—C52 | 7.9 (6) |
| C42—N4—C4—N3 | 14.2 (4) | C6—C5—C51—C52 | −150.8 (3) |
| C41—N4—C4—C5 | 17.2 (4) | C5—C51—C52—C53 | 3.5 (6) |
| C42—N4—C4—C5 | −166.9 (3) | C5—C51—C52—C55 | 175.3 (3) |
| N3—C4—C5—C51 | −133.3 (3) | C57—O56—C55—O55 | 1.5 (4) |
| N4—C4—C5—C51 | 47.8 (5) | C57—O56—C55—C52 | −177.9 (2) |
| N3—C4—C5—C6 | 26.3 (4) | C51—C52—C55—O55 | 5.5 (5) |
| N4—C4—C5—C6 | −152.6 (3) | C53—C52—C55—O55 | 177.9 (3) |
| C2—N1—C6—N6 | −178.5 (3) | C51—C52—C55—O56 | −175.2 (3) |
| C2—N1—C6—C5 | 5.4 (4) | C53—C52—C55—O56 | −2.8 (4) |
| C61—N6—C6—N1 | −12.2 (4) | C55—O56—C57—C58 | 163.4 (3) |
| C62—N6—C6—N1 | 153.5 (3) | | |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2A···N1i | 0.86 | 2.55 | 3.398 (4) | 170 |
| N2—H2B···O55ii | 0.86 | 2.17 | 2.966 (3) | 153 |
| Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) x, y, z+1. |
(II) 2-[(2-amino-4,6-di-1-piperidylpyrimidin-5-yl)methylene]malononitrile
top
Crystal data top
| C18H23N7 | Z = 4 |
| Mr = 337.43 | F(000) = 720 |
| Triclinic, P1 | Dx = 1.152 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.6960 (8) Å | Cell parameters from 8590 reflections |
| b = 14.752 (3) Å | θ = 3.9–27.5° |
| c = 15.418 (3) Å | µ = 0.07 mm−1 |
| α = 116.332 (13)° | T = 120 K |
| β = 96.794 (10)° | Block, colourless |
| γ = 93.587 (9)° | 0.62 × 0.38 × 0.32 mm |
| V = 1946.1 (6) Å3 | |
Data collection top
Bruker–Nonius KappaCCD
diffractometer | 8590 independent reflections |
| Radiation source: Bruker–Nonius FR591 rotating anode | 4805 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.049 |
| Detector resolution: 9.091 pixels mm-1 | θmax = 27.5°, θmin = 3.9° |
| ϕ and ω scans | h = −12→12 |
Absorption correction: multi-scan
(SADABS; Sheldrick, 2003) | k = −18→19 |
| Tmin = 0.962, Tmax = 0.977 | l = −19→20 |
| 38120 measured reflections | |
Refinement top
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.065 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.206 | H-atom parameters constrained |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0985P)2 + 0.7388P]
where P = (Fo2 + 2Fc2)/3 |
| 8590 reflections | (Δ/σ)max < 0.001 |
| 451 parameters | Δρmax = 0.31 e Å−3 |
| 0 restraints | Δρmin = −0.29 e Å−3 |
Crystal data top
| C18H23N7 | γ = 93.587 (9)° |
| Mr = 337.43 | V = 1946.1 (6) Å3 |
| Triclinic, P1 | Z = 4 |
| a = 9.6960 (8) Å | Mo Kα radiation |
| b = 14.752 (3) Å | µ = 0.07 mm−1 |
| c = 15.418 (3) Å | T = 120 K |
| α = 116.332 (13)° | 0.62 × 0.38 × 0.32 mm |
| β = 96.794 (10)° | |
Data collection top
Bruker–Nonius KappaCCD
diffractometer | 8590 independent reflections |
Absorption correction: multi-scan
(SADABS; Sheldrick, 2003) | 4805 reflections with I > 2σ(I) |
| Tmin = 0.962, Tmax = 0.977 | Rint = 0.049 |
| 38120 measured reflections | |
Refinement top
| R[F2 > 2σ(F2)] = 0.065 | 0 restraints |
| wR(F2) = 0.206 | H-atom parameters constrained |
| S = 1.10 | Δρmax = 0.31 e Å−3 |
| 8590 reflections | Δρmin = −0.29 e Å−3 |
| 451 parameters | |
Special details top
Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)
are estimated using the full covariance matrix. The cell e.s.d.'s are taken
into account individually in the estimation of e.s.d.'s in distances, angles
and torsion angles; correlations between e.s.d.'s in cell parameters are only
used when they are defined by crystal symmetry. An approximate (isotropic)
treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s.
planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) top| | x | y | z | Uiso*/Ueq | |
| N11 | 0.1453 (2) | 0.27967 (16) | 0.62366 (14) | 0.0216 (5) | |
| N12 | 0.0453 (2) | 0.23885 (17) | 0.46741 (15) | 0.0287 (5) | |
| H12A | 0.1140 | 0.2060 | 0.4455 | 0.034* | |
| H12B | −0.0200 | 0.2403 | 0.4253 | 0.034* | |
| N13 | −0.0488 (2) | 0.36072 (16) | 0.58977 (15) | 0.0242 (5) | |
| N14 | −0.1297 (2) | 0.48813 (16) | 0.71721 (15) | 0.0221 (5) | |
| N16 | 0.2347 (2) | 0.30988 (16) | 0.78053 (15) | 0.0227 (5) | |
| N154 | −0.3884 (2) | 0.39981 (19) | 0.76521 (18) | 0.0347 (6) | |
| N156 | −0.2001 (2) | 0.35445 (19) | 1.01184 (18) | 0.0331 (6) | |
| C12 | 0.0443 (3) | 0.29356 (19) | 0.56345 (18) | 0.0220 (6) | |
| C14 | −0.0543 (3) | 0.40955 (19) | 0.68562 (18) | 0.0205 (6) | |
| C15 | 0.0191 (3) | 0.37801 (19) | 0.75365 (18) | 0.0210 (6) | |
| C16 | 0.1364 (3) | 0.32394 (18) | 0.71857 (19) | 0.0205 (6) | |
| C142 | −0.2310 (3) | 0.5034 (2) | 0.64776 (19) | 0.0261 (6) | |
| H42A | −0.3234 | 0.5088 | 0.6697 | 0.031* | |
| H42B | −0.2416 | 0.4438 | 0.5823 | 0.031* | |
| C143 | −0.1838 (3) | 0.5991 (2) | 0.6402 (2) | 0.0318 (7) | |
| H43A | −0.2576 | 0.6109 | 0.5975 | 0.038* | |
| H43B | −0.0980 | 0.5901 | 0.6099 | 0.038* | |
| C144 | −0.1544 (3) | 0.6910 (2) | 0.7406 (2) | 0.0303 (7) | |
| H44A | −0.2434 | 0.7068 | 0.7664 | 0.036* | |
| H44B | −0.1137 | 0.7509 | 0.7348 | 0.036* | |
| C145 | −0.0536 (3) | 0.6708 (2) | 0.8114 (2) | 0.0286 (6) | |
| H45A | −0.0435 | 0.7291 | 0.8777 | 0.034* | |
| H45B | 0.0396 | 0.6657 | 0.7907 | 0.034* | |
| C146 | −0.1027 (3) | 0.57302 (19) | 0.81622 (18) | 0.0243 (6) | |
| H46A | −0.0298 | 0.5591 | 0.8578 | 0.029* | |
| H46B | −0.1892 | 0.5809 | 0.8457 | 0.029* | |
| C151 | −0.0341 (3) | 0.37128 (18) | 0.83164 (18) | 0.0222 (6) | |
| H151 | 0.0324 | 0.3562 | 0.8722 | 0.027* | |
| C152 | −0.1645 (3) | 0.38224 (19) | 0.86154 (18) | 0.0218 (6) | |
| C153 | −0.2859 (3) | 0.3934 (2) | 0.8081 (2) | 0.0256 (6) | |
| C155 | −0.1853 (3) | 0.3664 (2) | 0.9440 (2) | 0.0241 (6) | |
| C162 | 0.3265 (3) | 0.2314 (2) | 0.7419 (2) | 0.0262 (6) | |
| H62A | 0.4131 | 0.2610 | 0.7306 | 0.031* | |
| H62B | 0.2789 | 0.1757 | 0.6783 | 0.031* | |
| C163 | 0.3637 (3) | 0.1892 (2) | 0.8143 (2) | 0.0308 (7) | |
| H63A | 0.2782 | 0.1521 | 0.8188 | 0.037* | |
| H63B | 0.4317 | 0.1400 | 0.7899 | 0.037* | |
| C164 | 0.4260 (3) | 0.2730 (2) | 0.9156 (2) | 0.0347 (7) | |
| H64A | 0.5170 | 0.3053 | 0.9129 | 0.042* | |
| H64B | 0.4430 | 0.2434 | 0.9617 | 0.042* | |
| C165 | 0.3266 (3) | 0.3536 (2) | 0.9525 (2) | 0.0318 (7) | |
| H65A | 0.2391 | 0.3232 | 0.9618 | 0.038* | |
| H65B | 0.3709 | 0.4104 | 1.0164 | 0.038* | |
| C166 | 0.2936 (3) | 0.3933 (2) | 0.87794 (19) | 0.0245 (6) | |
| H66A | 0.2258 | 0.4432 | 0.9001 | 0.029* | |
| H66B | 0.3804 | 0.4290 | 0.8734 | 0.029* | |
| N21 | 0.3141 (2) | 0.16895 (16) | 0.39431 (15) | 0.0225 (5) | |
| N22 | 0.4239 (2) | 0.27580 (18) | 0.54959 (15) | 0.0311 (6) | |
| H22A | 0.3508 | 0.2593 | 0.5687 | 0.037* | |
| H22B | 0.4893 | 0.3223 | 0.5907 | 0.037* | |
| N23 | 0.5226 (2) | 0.28412 (16) | 0.42548 (15) | 0.0232 (5) | |
| N24 | 0.6120 (2) | 0.29151 (16) | 0.29581 (15) | 0.0226 (5) | |
| N26 | 0.2154 (2) | 0.04855 (15) | 0.23860 (15) | 0.0222 (5) | |
| N254 | 0.8560 (3) | 0.16240 (19) | 0.26326 (19) | 0.0358 (6) | |
| N256 | 0.6582 (2) | −0.10128 (18) | 0.00068 (17) | 0.0320 (6) | |
| C22 | 0.4228 (3) | 0.24072 (19) | 0.45319 (19) | 0.0230 (6) | |
| C24 | 0.5270 (3) | 0.24128 (19) | 0.32853 (18) | 0.0220 (6) | |
| C25 | 0.4441 (3) | 0.14492 (19) | 0.26252 (18) | 0.0211 (6) | |
| C26 | 0.3216 (3) | 0.12164 (19) | 0.29805 (18) | 0.0216 (6) | |
| C242 | 0.7198 (3) | 0.3752 (2) | 0.36422 (19) | 0.0261 (6) | |
| H42C | 0.8119 | 0.3615 | 0.3428 | 0.031* | |
| H42D | 0.7259 | 0.3793 | 0.4304 | 0.031* | |
| C243 | 0.6859 (3) | 0.4760 (2) | 0.3687 (2) | 0.0292 (7) | |
| H43C | 0.5988 | 0.4934 | 0.3966 | 0.035* | |
| H43D | 0.7627 | 0.5307 | 0.4119 | 0.035* | |
| C244 | 0.6675 (3) | 0.4692 (2) | 0.26692 (19) | 0.0275 (6) | |
| H44C | 0.7589 | 0.4626 | 0.2435 | 0.033* | |
| H44D | 0.6355 | 0.5328 | 0.2699 | 0.033* | |
| C245 | 0.5616 (3) | 0.3784 (2) | 0.19463 (19) | 0.0259 (6) | |
| H45C | 0.4669 | 0.3914 | 0.2112 | 0.031* | |
| H45D | 0.5615 | 0.3714 | 0.1277 | 0.031* | |
| C246 | 0.5930 (3) | 0.2788 (2) | 0.19524 (18) | 0.0235 (6) | |
| H46C | 0.5147 | 0.2243 | 0.1545 | 0.028* | |
| H46D | 0.6791 | 0.2583 | 0.1668 | 0.028* | |
| C251 | 0.4928 (3) | 0.06654 (19) | 0.18434 (18) | 0.0214 (6) | |
| H251 | 0.4222 | 0.0121 | 0.1417 | 0.026* | |
| C252 | 0.6244 (3) | 0.05412 (19) | 0.15815 (18) | 0.0222 (6) | |
| C253 | 0.7509 (3) | 0.1163 (2) | 0.2168 (2) | 0.0259 (6) | |
| C255 | 0.6434 (3) | −0.0320 (2) | 0.0711 (2) | 0.0236 (6) | |
| C262 | 0.1141 (3) | 0.0053 (2) | 0.2785 (2) | 0.0279 (6) | |
| H62C | 0.0285 | 0.0394 | 0.2836 | 0.033* | |
| H62D | 0.1551 | 0.0167 | 0.3451 | 0.033* | |
| C263 | 0.0769 (3) | −0.1088 (2) | 0.2110 (2) | 0.0315 (7) | |
| H63C | 0.1609 | −0.1433 | 0.2122 | 0.038* | |
| H63D | 0.0043 | −0.1371 | 0.2356 | 0.038* | |
| C264 | 0.0231 (3) | −0.1309 (2) | 0.1060 (2) | 0.0346 (7) | |
| H64C | −0.0670 | −0.1035 | 0.1032 | 0.042* | |
| H64D | 0.0065 | −0.2056 | 0.0637 | 0.042* | |
| C265 | 0.1303 (3) | −0.0819 (2) | 0.0682 (2) | 0.0306 (7) | |
| H65C | 0.2167 | −0.1150 | 0.0635 | 0.037* | |
| H65D | 0.0911 | −0.0915 | 0.0019 | 0.037* | |
| C266 | 0.1647 (3) | 0.0317 (2) | 0.13820 (18) | 0.0258 (6) | |
| H66C | 0.2373 | 0.0625 | 0.1157 | 0.031* | |
| H66D | 0.0799 | 0.0656 | 0.1378 | 0.031* | |
Atomic displacement parameters (Å2) top| | U11 | U22 | U33 | U12 | U13 | U23 |
| N11 | 0.0211 (12) | 0.0243 (12) | 0.0157 (11) | 0.0017 (9) | 0.0020 (9) | 0.0061 (9) |
| N12 | 0.0270 (13) | 0.0345 (13) | 0.0184 (12) | 0.0095 (10) | 0.0018 (10) | 0.0064 (10) |
| N13 | 0.0239 (12) | 0.0265 (12) | 0.0204 (12) | 0.0062 (10) | 0.0044 (9) | 0.0085 (10) |
| N14 | 0.0236 (12) | 0.0216 (11) | 0.0187 (11) | 0.0039 (9) | 0.0022 (9) | 0.0074 (9) |
| N16 | 0.0222 (12) | 0.0241 (12) | 0.0201 (12) | 0.0043 (9) | 0.0030 (9) | 0.0086 (10) |
| N154 | 0.0226 (13) | 0.0415 (15) | 0.0419 (15) | −0.0035 (11) | −0.0007 (12) | 0.0232 (13) |
| N156 | 0.0243 (13) | 0.0448 (15) | 0.0312 (14) | 0.0049 (11) | 0.0056 (11) | 0.0178 (12) |
| C12 | 0.0204 (14) | 0.0201 (13) | 0.0226 (14) | −0.0004 (11) | 0.0027 (11) | 0.0078 (11) |
| C14 | 0.0175 (13) | 0.0192 (13) | 0.0205 (14) | −0.0020 (10) | 0.0017 (11) | 0.0062 (11) |
| C15 | 0.0221 (14) | 0.0212 (13) | 0.0165 (13) | 0.0015 (11) | 0.0007 (11) | 0.0064 (11) |
| C16 | 0.0183 (13) | 0.0179 (13) | 0.0247 (14) | −0.0014 (10) | 0.0010 (11) | 0.0103 (11) |
| C142 | 0.0230 (14) | 0.0320 (15) | 0.0196 (14) | 0.0086 (12) | −0.0001 (11) | 0.0087 (12) |
| C143 | 0.0319 (16) | 0.0380 (17) | 0.0336 (16) | 0.0134 (13) | 0.0071 (13) | 0.0219 (14) |
| C144 | 0.0273 (15) | 0.0284 (15) | 0.0377 (17) | 0.0033 (12) | 0.0039 (13) | 0.0176 (14) |
| C145 | 0.0302 (16) | 0.0213 (14) | 0.0305 (16) | 0.0001 (12) | 0.0028 (12) | 0.0095 (12) |
| C146 | 0.0250 (14) | 0.0235 (14) | 0.0204 (14) | 0.0040 (11) | 0.0018 (11) | 0.0069 (12) |
| C151 | 0.0260 (14) | 0.0191 (13) | 0.0173 (13) | −0.0001 (11) | −0.0013 (11) | 0.0062 (11) |
| C152 | 0.0187 (14) | 0.0242 (14) | 0.0215 (14) | 0.0023 (11) | 0.0029 (11) | 0.0097 (11) |
| C153 | 0.0260 (15) | 0.0237 (14) | 0.0254 (15) | 0.0000 (12) | 0.0049 (12) | 0.0097 (12) |
| C155 | 0.0174 (14) | 0.0292 (15) | 0.0245 (15) | 0.0026 (11) | 0.0039 (11) | 0.0111 (12) |
| C162 | 0.0245 (15) | 0.0276 (15) | 0.0291 (15) | 0.0086 (12) | 0.0081 (12) | 0.0137 (13) |
| C163 | 0.0276 (16) | 0.0331 (16) | 0.0370 (17) | 0.0053 (13) | 0.0040 (13) | 0.0208 (14) |
| C164 | 0.0344 (17) | 0.0414 (17) | 0.0357 (17) | 0.0016 (14) | −0.0016 (14) | 0.0265 (15) |
| C165 | 0.0310 (16) | 0.0409 (17) | 0.0216 (15) | −0.0035 (13) | −0.0024 (12) | 0.0151 (13) |
| C166 | 0.0205 (14) | 0.0251 (14) | 0.0254 (15) | 0.0007 (11) | 0.0006 (11) | 0.0103 (12) |
| N21 | 0.0215 (12) | 0.0246 (12) | 0.0193 (12) | 0.0012 (9) | 0.0040 (9) | 0.0081 (10) |
| N22 | 0.0268 (13) | 0.0397 (14) | 0.0192 (12) | −0.0077 (11) | 0.0022 (10) | 0.0086 (11) |
| N23 | 0.0231 (12) | 0.0232 (11) | 0.0194 (12) | −0.0009 (9) | 0.0029 (9) | 0.0068 (10) |
| N24 | 0.0241 (12) | 0.0245 (12) | 0.0172 (11) | −0.0042 (9) | −0.0009 (9) | 0.0097 (10) |
| N26 | 0.0203 (11) | 0.0215 (11) | 0.0223 (12) | −0.0034 (9) | 0.0028 (9) | 0.0087 (10) |
| N254 | 0.0291 (14) | 0.0367 (14) | 0.0402 (15) | 0.0059 (12) | −0.0005 (12) | 0.0176 (12) |
| N256 | 0.0337 (14) | 0.0319 (14) | 0.0289 (14) | 0.0054 (11) | 0.0080 (11) | 0.0116 (12) |
| C22 | 0.0244 (14) | 0.0223 (14) | 0.0220 (14) | 0.0049 (11) | 0.0057 (11) | 0.0093 (12) |
| C24 | 0.0204 (14) | 0.0249 (14) | 0.0211 (14) | 0.0020 (11) | 0.0021 (11) | 0.0113 (12) |
| C25 | 0.0211 (14) | 0.0231 (13) | 0.0173 (13) | 0.0009 (11) | 0.0010 (11) | 0.0085 (11) |
| C26 | 0.0245 (14) | 0.0206 (13) | 0.0208 (14) | 0.0034 (11) | 0.0034 (11) | 0.0105 (11) |
| C242 | 0.0216 (14) | 0.0305 (15) | 0.0213 (14) | −0.0064 (12) | −0.0038 (11) | 0.0105 (12) |
| C243 | 0.0275 (15) | 0.0254 (15) | 0.0261 (15) | −0.0066 (12) | 0.0013 (12) | 0.0060 (12) |
| C244 | 0.0269 (15) | 0.0225 (14) | 0.0326 (16) | 0.0001 (12) | 0.0045 (12) | 0.0125 (12) |
| C245 | 0.0273 (15) | 0.0276 (15) | 0.0221 (14) | 0.0024 (12) | 0.0023 (12) | 0.0111 (12) |
| C246 | 0.0254 (14) | 0.0247 (14) | 0.0181 (14) | −0.0015 (11) | 0.0013 (11) | 0.0088 (11) |
| C251 | 0.0226 (14) | 0.0230 (14) | 0.0180 (13) | −0.0031 (11) | −0.0022 (11) | 0.0107 (11) |
| C252 | 0.0252 (15) | 0.0241 (14) | 0.0184 (14) | 0.0020 (11) | 0.0035 (11) | 0.0106 (12) |
| C253 | 0.0230 (15) | 0.0277 (15) | 0.0264 (15) | 0.0075 (12) | 0.0034 (12) | 0.0115 (12) |
| C255 | 0.0222 (14) | 0.0267 (15) | 0.0233 (15) | 0.0033 (12) | 0.0034 (12) | 0.0126 (13) |
| C262 | 0.0267 (15) | 0.0255 (14) | 0.0296 (16) | 0.0000 (12) | 0.0078 (12) | 0.0105 (12) |
| C263 | 0.0355 (17) | 0.0237 (14) | 0.0333 (16) | −0.0015 (12) | 0.0089 (13) | 0.0111 (13) |
| C264 | 0.0285 (16) | 0.0249 (15) | 0.0396 (18) | −0.0042 (12) | 0.0021 (13) | 0.0068 (13) |
| C265 | 0.0300 (16) | 0.0310 (15) | 0.0203 (14) | −0.0017 (12) | −0.0002 (12) | 0.0043 (12) |
| C266 | 0.0243 (14) | 0.0268 (15) | 0.0220 (14) | 0.0011 (12) | −0.0006 (12) | 0.0084 (12) |
Geometric parameters (Å, º) top
| N11—C16 | 1.328 (3) | N21—C26 | 1.345 (3) |
| N11—C12 | 1.357 (3) | N21—C22 | 1.356 (3) |
| N12—C12 | 1.337 (3) | N22—C22 | 1.338 (3) |
| N12—H12A | 0.8600 | N22—H22A | 0.8600 |
| N12—H12B | 0.8601 | N22—H22B | 0.8600 |
| N13—C14 | 1.336 (3) | N23—C22 | 1.339 (3) |
| N13—C12 | 1.342 (3) | N23—C24 | 1.348 (3) |
| N14—C14 | 1.343 (3) | N24—C24 | 1.355 (3) |
| N14—C146 | 1.461 (3) | N24—C246 | 1.464 (3) |
| N14—C142 | 1.468 (3) | N24—C242 | 1.467 (3) |
| N16—C16 | 1.360 (3) | N26—C26 | 1.351 (3) |
| N16—C162 | 1.460 (3) | N26—C266 | 1.471 (3) |
| N16—C166 | 1.472 (3) | N26—C262 | 1.471 (3) |
| N154—C153 | 1.160 (3) | N254—C253 | 1.150 (3) |
| N156—C155 | 1.158 (3) | N256—C255 | 1.147 (3) |
| C14—C15 | 1.456 (4) | C24—C25 | 1.443 (4) |
| C15—C151 | 1.403 (4) | C25—C251 | 1.407 (4) |
| C15—C16 | 1.448 (4) | C25—C26 | 1.449 (4) |
| C142—C143 | 1.514 (4) | C242—C243 | 1.516 (4) |
| C142—H42A | 0.9900 | C242—H42C | 0.9900 |
| C142—H42B | 0.9900 | C242—H42D | 0.9900 |
| C143—C144 | 1.517 (4) | C243—C244 | 1.515 (4) |
| C143—H43A | 0.9900 | C243—H43C | 0.9900 |
| C143—H43B | 0.9900 | C243—H43D | 0.9900 |
| C144—C145 | 1.520 (4) | C244—C245 | 1.525 (4) |
| C144—H44A | 0.9900 | C244—H44C | 0.9900 |
| C144—H44B | 0.9900 | C244—H44D | 0.9900 |
| C145—C146 | 1.526 (4) | C245—C246 | 1.523 (4) |
| C145—H45A | 0.9900 | C245—H45C | 0.9900 |
| C145—H45B | 0.9900 | C245—H45D | 0.9900 |
| C146—H46A | 0.9900 | C246—H46C | 0.9900 |
| C146—H46B | 0.9900 | C246—H46D | 0.9900 |
| C151—C152 | 1.391 (4) | C251—C252 | 1.382 (4) |
| C151—H151 | 0.9500 | C251—H251 | 0.9500 |
| C152—C153 | 1.417 (4) | C252—C253 | 1.423 (4) |
| C152—C155 | 1.425 (4) | C252—C255 | 1.423 (4) |
| C162—C163 | 1.522 (4) | C262—C263 | 1.526 (4) |
| C162—H62A | 0.9900 | C262—H62C | 0.9900 |
| C162—H62B | 0.9900 | C262—H62D | 0.9900 |
| C163—C164 | 1.518 (4) | C263—C264 | 1.519 (4) |
| C163—H63A | 0.9900 | C263—H63C | 0.9900 |
| C163—H63B | 0.9900 | C263—H63D | 0.9900 |
| C164—C165 | 1.529 (4) | C264—C265 | 1.539 (4) |
| C164—H64A | 0.9900 | C264—H64C | 0.9900 |
| C164—H64B | 0.9900 | C264—H64D | 0.9900 |
| C165—C166 | 1.517 (4) | C265—C266 | 1.524 (4) |
| C165—H65A | 0.9900 | C265—H65C | 0.9900 |
| C165—H65B | 0.9900 | C265—H65D | 0.9900 |
| C166—H66A | 0.9900 | C266—H66C | 0.9900 |
| C166—H66B | 0.9900 | C266—H66D | 0.9900 |
| | | |
| C16—N11—C12 | 116.4 (2) | C26—N21—C22 | 116.5 (2) |
| C12—N12—H12A | 122.0 | C22—N22—H22A | 118.2 |
| C12—N12—H12B | 120.1 | C22—N22—H22B | 119.8 |
| H12A—N12—H12B | 117.7 | H22A—N22—H22B | 120.9 |
| C14—N13—C12 | 116.0 (2) | C22—N23—C24 | 116.0 (2) |
| C14—N14—C146 | 124.2 (2) | C24—N24—C246 | 124.3 (2) |
| C14—N14—C142 | 120.7 (2) | C24—N24—C242 | 120.9 (2) |
| C146—N14—C142 | 114.3 (2) | C246—N24—C242 | 114.2 (2) |
| C16—N16—C162 | 120.3 (2) | C26—N26—C266 | 122.3 (2) |
| C16—N16—C166 | 121.3 (2) | C26—N26—C262 | 121.3 (2) |
| C162—N16—C166 | 113.6 (2) | C266—N26—C262 | 113.4 (2) |
| N12—C12—N13 | 117.1 (2) | N22—C22—N23 | 117.2 (2) |
| N12—C12—N11 | 115.5 (2) | N22—C22—N21 | 115.5 (2) |
| N13—C12—N11 | 127.2 (2) | N23—C22—N21 | 127.2 (2) |
| N13—C14—N14 | 118.1 (2) | N23—C24—N24 | 117.7 (2) |
| N13—C14—C15 | 120.8 (2) | N23—C24—C25 | 120.7 (2) |
| N14—C14—C15 | 121.1 (2) | N24—C24—C25 | 121.6 (2) |
| C151—C15—C16 | 118.5 (2) | C251—C25—C24 | 124.0 (2) |
| C151—C15—C14 | 125.5 (2) | C251—C25—C26 | 119.5 (2) |
| C16—C15—C14 | 113.6 (2) | C24—C25—C26 | 114.4 (2) |
| N11—C16—N16 | 117.8 (2) | N21—C26—N26 | 117.2 (2) |
| N11—C16—C15 | 121.0 (2) | N21—C26—C25 | 120.2 (2) |
| N16—C16—C15 | 121.0 (2) | N26—C26—C25 | 122.4 (2) |
| N14—C142—C143 | 111.1 (2) | N24—C242—C243 | 110.9 (2) |
| N14—C142—H42A | 109.4 | N24—C242—H42C | 109.5 |
| C143—C142—H42A | 109.4 | C243—C242—H42C | 109.5 |
| N14—C142—H42B | 109.4 | N24—C242—H42D | 109.5 |
| C143—C142—H42B | 109.4 | C243—C242—H42D | 109.5 |
| H42A—C142—H42B | 108.0 | H42C—C242—H42D | 108.1 |
| C142—C143—C144 | 110.9 (2) | C244—C243—C242 | 110.2 (2) |
| C142—C143—H43A | 109.5 | C244—C243—H43C | 109.6 |
| C144—C143—H43A | 109.5 | C242—C243—H43C | 109.6 |
| C142—C143—H43B | 109.5 | C244—C243—H43D | 109.6 |
| C144—C143—H43B | 109.5 | C242—C243—H43D | 109.6 |
| H43A—C143—H43B | 108.0 | H43C—C243—H43D | 108.1 |
| C143—C144—C145 | 110.8 (2) | C243—C244—C245 | 111.5 (2) |
| C143—C144—H44A | 109.5 | C243—C244—H44C | 109.3 |
| C145—C144—H44A | 109.5 | C245—C244—H44C | 109.3 |
| C143—C144—H44B | 109.5 | C243—C244—H44D | 109.3 |
| C145—C144—H44B | 109.5 | C245—C244—H44D | 109.3 |
| H44A—C144—H44B | 108.1 | H44C—C244—H44D | 108.0 |
| C144—C145—C146 | 112.5 (2) | C246—C245—C244 | 112.7 (2) |
| C144—C145—H45A | 109.1 | C246—C245—H45C | 109.0 |
| C146—C145—H45A | 109.1 | C244—C245—H45C | 109.0 |
| C144—C145—H45B | 109.1 | C246—C245—H45D | 109.0 |
| C146—C145—H45B | 109.1 | C244—C245—H45D | 109.0 |
| H45A—C145—H45B | 107.8 | H45C—C245—H45D | 107.8 |
| N14—C146—C145 | 109.3 (2) | N24—C246—C245 | 110.0 (2) |
| N14—C146—H46A | 109.8 | N24—C246—H46C | 109.7 |
| C145—C146—H46A | 109.8 | C245—C246—H46C | 109.7 |
| N14—C146—H46B | 109.8 | N24—C246—H46D | 109.7 |
| C145—C146—H46B | 109.8 | C245—C246—H46D | 109.7 |
| H46A—C146—H46B | 108.3 | H46C—C246—H46D | 108.2 |
| C152—C151—C15 | 132.9 (2) | C252—C251—C25 | 131.9 (2) |
| C152—C151—H151 | 113.5 | C252—C251—H251 | 114.1 |
| C15—C151—H151 | 113.5 | C25—C251—H251 | 114.1 |
| C151—C152—C153 | 124.9 (2) | C251—C252—C253 | 125.1 (2) |
| C151—C152—C155 | 118.5 (2) | C251—C252—C255 | 120.2 (2) |
| C153—C152—C155 | 116.0 (2) | C253—C252—C255 | 114.4 (2) |
| N154—C153—C152 | 177.2 (3) | N254—C253—C252 | 176.7 (3) |
| N156—C155—C152 | 178.9 (3) | N256—C255—C252 | 179.7 (3) |
| N16—C162—C163 | 109.8 (2) | N26—C262—C263 | 109.2 (2) |
| N16—C162—H62A | 109.7 | N26—C262—H62C | 109.8 |
| C163—C162—H62A | 109.7 | C263—C262—H62C | 109.8 |
| N16—C162—H62B | 109.7 | N26—C262—H62D | 109.8 |
| C163—C162—H62B | 109.7 | C263—C262—H62D | 109.8 |
| H62A—C162—H62B | 108.2 | H62C—C262—H62D | 108.3 |
| C164—C163—C162 | 111.8 (2) | C264—C263—C262 | 112.0 (2) |
| C164—C163—H63A | 109.2 | C264—C263—H63C | 109.2 |
| C162—C163—H63A | 109.2 | C262—C263—H63C | 109.2 |
| C164—C163—H63B | 109.2 | C264—C263—H63D | 109.2 |
| C162—C163—H63B | 109.2 | C262—C263—H63D | 109.2 |
| H63A—C163—H63B | 107.9 | H63C—C263—H63D | 107.9 |
| C163—C164—C165 | 110.3 (2) | C263—C264—C265 | 110.3 (2) |
| C163—C164—H64A | 109.6 | C263—C264—H64C | 109.6 |
| C165—C164—H64A | 109.6 | C265—C264—H64C | 109.6 |
| C163—C164—H64B | 109.6 | C263—C264—H64D | 109.6 |
| C165—C164—H64B | 109.6 | C265—C264—H64D | 109.6 |
| H64A—C164—H64B | 108.1 | H64C—C264—H64D | 108.1 |
| C166—C165—C164 | 109.1 (2) | C266—C265—C264 | 109.5 (2) |
| C166—C165—H65A | 109.9 | C266—C265—H65C | 109.8 |
| C164—C165—H65A | 109.9 | C264—C265—H65C | 109.8 |
| C166—C165—H65B | 109.9 | C266—C265—H65D | 109.8 |
| C164—C165—H65B | 109.9 | C264—C265—H65D | 109.8 |
| H65A—C165—H65B | 108.3 | H65C—C265—H65D | 108.2 |
| N16—C166—C165 | 111.3 (2) | N26—C266—C265 | 111.0 (2) |
| N16—C166—H66A | 109.4 | N26—C266—H66C | 109.4 |
| C165—C166—H66A | 109.4 | C265—C266—H66C | 109.4 |
| N16—C166—H66B | 109.4 | N26—C266—H66D | 109.4 |
| C165—C166—H66B | 109.4 | C265—C266—H66D | 109.4 |
| H66A—C166—H66B | 108.0 | H66C—C266—H66D | 108.0 |
| | | |
| C14—N13—C12—N12 | 175.7 (2) | C24—N23—C22—N22 | 174.1 (2) |
| C14—N13—C12—N11 | −9.5 (4) | C24—N23—C22—N21 | −10.5 (4) |
| C16—N11—C12—N12 | −172.4 (2) | C26—N21—C22—N22 | −170.4 (2) |
| C16—N11—C12—N13 | 12.7 (4) | C26—N21—C22—N23 | 14.2 (4) |
| C12—N13—C14—N14 | 170.1 (2) | C22—N23—C24—N24 | 170.3 (2) |
| C12—N13—C14—C15 | −10.1 (3) | C22—N23—C24—C25 | −9.9 (3) |
| C146—N14—C14—N13 | −153.5 (2) | C246—N24—C24—N23 | −156.3 (2) |
| C142—N14—C14—N13 | 15.4 (4) | C242—N24—C24—N23 | 14.6 (3) |
| C146—N14—C14—C15 | 26.7 (4) | C246—N24—C24—C25 | 24.0 (4) |
| C142—N14—C14—C15 | −164.4 (2) | C242—N24—C24—C25 | −165.2 (2) |
| N13—C14—C15—C151 | −138.2 (3) | N23—C24—C25—C251 | −138.9 (3) |
| N14—C14—C15—C151 | 41.6 (4) | N24—C24—C25—C251 | 40.8 (4) |
| N13—C14—C15—C16 | 24.0 (3) | N23—C24—C25—C26 | 24.1 (3) |
| N14—C14—C15—C16 | −156.2 (2) | N24—C24—C25—C26 | −156.2 (2) |
| C12—N11—C16—N16 | 178.8 (2) | C22—N21—C26—N26 | 178.8 (2) |
| C12—N11—C16—C15 | 4.0 (3) | C22—N21—C26—C25 | 2.7 (3) |
| C162—N16—C16—N11 | −13.3 (3) | C266—N26—C26—N21 | 144.9 (2) |
| C166—N16—C16—N11 | 140.7 (2) | C262—N26—C26—N21 | −14.2 (3) |
| C162—N16—C16—C15 | 161.6 (2) | C266—N26—C26—C25 | −39.1 (4) |
| C166—N16—C16—C15 | −44.5 (3) | C262—N26—C26—C25 | 161.8 (2) |
| C151—C15—C16—N11 | 142.9 (2) | C251—C25—C26—N21 | 143.7 (2) |
| C14—C15—C16—N11 | −20.6 (3) | C24—C25—C26—N21 | −20.1 (3) |
| C151—C15—C16—N16 | −31.7 (3) | C251—C25—C26—N26 | −32.2 (4) |
| C14—C15—C16—N16 | 164.7 (2) | C24—C25—C26—N26 | 164.0 (2) |
| C14—N14—C142—C143 | −112.4 (3) | C24—N24—C242—C243 | −113.0 (3) |
| C146—N14—C142—C143 | 57.6 (3) | C246—N24—C242—C243 | 58.7 (3) |
| N14—C142—C143—C144 | −54.2 (3) | N24—C242—C243—C244 | −55.8 (3) |
| C142—C143—C144—C145 | 52.8 (3) | C242—C243—C244—C245 | 52.9 (3) |
| C143—C144—C145—C146 | −53.3 (3) | C243—C244—C245—C246 | −51.6 (3) |
| C14—N14—C146—C145 | 113.2 (3) | C24—N24—C246—C245 | 115.8 (3) |
| C142—N14—C146—C145 | −56.3 (3) | C242—N24—C246—C245 | −55.5 (3) |
| C144—C145—C146—N14 | 53.8 (3) | C244—C245—C246—N24 | 51.3 (3) |
| C16—C15—C151—C152 | −155.2 (3) | C24—C25—C251—C252 | 8.9 (4) |
| C14—C15—C151—C152 | 6.2 (5) | C26—C25—C251—C252 | −153.3 (3) |
| C15—C151—C152—C153 | 7.4 (5) | C25—C251—C252—C253 | 9.7 (5) |
| C15—C151—C152—C155 | 177.7 (3) | C25—C251—C252—C255 | −176.9 (3) |
| C16—N16—C162—C163 | −148.1 (2) | C26—N26—C262—C263 | −141.8 (2) |
| C166—N16—C162—C163 | 56.0 (3) | C266—N26—C262—C263 | 57.4 (3) |
| N16—C162—C163—C164 | −54.6 (3) | N26—C262—C263—C264 | −55.4 (3) |
| C162—C163—C164—C165 | 55.5 (3) | C262—C263—C264—C265 | 55.3 (3) |
| C163—C164—C165—C166 | −55.5 (3) | C263—C264—C265—C266 | −54.6 (3) |
| C16—N16—C166—C165 | 146.1 (2) | C26—N26—C266—C265 | 140.3 (2) |
| C162—N16—C166—C165 | −58.3 (3) | C262—N26—C266—C265 | −59.1 (3) |
| C164—C165—C166—N16 | 56.4 (3) | C264—C265—C266—N26 | 56.1 (3) |
Hydrogen-bond geometry (Å, º) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N12—H12A···N21 | 0.86 | 2.19 | 3.021 (3) | 162 |
| N12—H12B···N254i | 0.86 | 2.36 | 3.126 (3) | 148 |
| N22—H22A···N11 | 0.86 | 2.24 | 3.049 (3) | 156 |
| N22—H22B···N154ii | 0.86 | 2.51 | 3.235 (3) | 143 |
| C162—H62A···N154ii | 0.99 | 2.54 | 3.472 (4) | 156 |
| C164—H64B···N256iii | 0.99 | 2.58 | 3.412 (4) | 142 |
| C246—H46C···N256iv | 0.99 | 2.58 | 3.515 (4) | 158 |
| C266—H66C···N256iv | 0.99 | 2.42 | 3.345 (4) | 156 |
| Symmetry codes: (i) x−1, y, z; (ii) x+1, y, z; (iii) −x+1, −y, −z+1; (iv) −x+1, −y, −z. |
Experimental details
| | (I) | (II) |
| Crystal data |
| Chemical formula | C14H20N6O2 | C18H23N7 |
| Mr | 304.36 | 337.43 |
| Crystal system, space group | Triclinic, P1 | Triclinic, P1 |
| Temperature (K) | 120 | 120 |
| a, b, c (Å) | 8.5570 (15), 9.893 (2), 9.9739 (18) | 9.6960 (8), 14.752 (3), 15.418 (3) |
| α, β, γ (°) | 87.534 (13), 77.251 (15), 67.431 (14) | 116.332 (13), 96.794 (10), 93.587 (9) |
| V (Å3) | 759.6 (3) | 1946.1 (6) |
| Z | 2 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.09 | 0.07 |
| Crystal size (mm) | 0.37 × 0.34 × 0.15 | 0.62 × 0.38 × 0.32 |
| |
| Data collection |
| Diffractometer | Bruker–Nonius KappaCCD
diffractometer | Bruker–Nonius KappaCCD
diffractometer |
| Absorption correction | Multi-scan
(SADABS; Sheldrick, 2003) | Multi-scan
(SADABS; Sheldrick, 2003) |
| Tmin, Tmax | 0.971, 0.986 | 0.962, 0.977 |
No. of measured, independent and
observed [I > 2σ(I)] reflections | 18938, 3468, 1715 | 38120, 8590, 4805 |
| Rint | 0.085 | 0.049 |
| (sin θ/λ)max (Å−1) | 0.650 | 0.650 |
| |
| Refinement |
| R[F2 > 2σ(F2)], wR(F2), S | 0.069, 0.236, 1.03 | 0.065, 0.206, 1.10 |
| No. of reflections | 3468 | 8590 |
| No. of parameters | 204 | 451 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.44, −0.37 | 0.31, −0.29 |
Hydrogen-bond geometry (Å, º) for (I) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2A···N1i | 0.86 | 2.55 | 3.398 (4) | 170 |
| N2—H2B···O55ii | 0.86 | 2.17 | 2.966 (3) | 153 |
| Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) x, y, z+1. |
Hydrogen-bond geometry (Å, º) for (II) top
| D—H···A | D—H | H···A | D···A | D—H···A |
| N12—H12A···N21 | 0.86 | 2.19 | 3.021 (3) | 162 |
| N12—H12B···N254i | 0.86 | 2.36 | 3.126 (3) | 148 |
| N22—H22A···N11 | 0.86 | 2.24 | 3.049 (3) | 156 |
| N22—H22B···N154ii | 0.86 | 2.51 | 3.235 (3) | 143 |
| C162—H62A···N154ii | 0.99 | 2.54 | 3.472 (4) | 156 |
| C164—H64B···N256iii | 0.99 | 2.58 | 3.412 (4) | 142 |
| C246—H46C···N256iv | 0.99 | 2.58 | 3.515 (4) | 158 |
| C266—H66C···N256iv | 0.99 | 2.42 | 3.345 (4) | 156 |
| Symmetry codes: (i) x−1, y, z; (ii) x+1, y, z; (iii) −x+1, −y, −z+1; (iv) −x+1, −y, −z. |
Ring-puckering parameters (Å, °) for the pyrimidine rings in compounds (I)
and (II) top| Parameter | (I) | (II), molecule 1 | (II) molecule 2 |
| Q | 0.248 (3) | 0.218 (3) | 0.221 (3) |
| θ | 74.4 (7) | 73.0 (8) | 75.0 (8) |
| ϕ | 234.6 (8) | 230.9 (8) | 229.7 (8) |

 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
The full text of this article is available to subscribers to the journal.
If you have already registered and are using a computer listed in your registration details, please email
support@iucr.org for assistance.
 N and N-H
N and N-H O hydrogen bonds into chains of edge-fused R22(8) and R44(20) rings, while the two independent molecules in (II) are linked by four N-H
O hydrogen bonds into chains of edge-fused R22(8) and R44(20) rings, while the two independent molecules in (II) are linked by four N-H N hydrogen bonds into chains of edge-fused R22(8) and R22(20) rings. This study illustrates both the readiness with which highly-substituted pyrimidine rings can be distorted from planarity and the significant differences between the supramolecular aggregation in two rather similar compounds.
N hydrogen bonds into chains of edge-fused R22(8) and R22(20) rings. This study illustrates both the readiness with which highly-substituted pyrimidine rings can be distorted from planarity and the significant differences between the supramolecular aggregation in two rather similar compounds.
 Subscribe to Acta Crystallographica Section C: Structural Chemistry
Subscribe to Acta Crystallographica Section C: Structural Chemistry
 journal menu
journal menu








![[Figure 1]](https://journals.iucr.org/c/issues/2008/03/00/sk3200/sk3200fig1thm.gif)
![[Figure 2]](https://journals.iucr.org/c/issues/2008/03/00/sk3200/sk3200fig2thm.gif)
![[Figure 3]](https://journals.iucr.org/c/issues/2008/03/00/sk3200/sk3200fig3thm.gif)
![[Figure 4]](https://journals.iucr.org/c/issues/2008/03/00/sk3200/sk3200fig4thm.gif)




As part of a programme for the synthesis of new heterocyclic compounds with potential biological activity, we have investigated the functionalization at position C-5 in pyrimidines via modification of 4,6-dichloro-5-formylpyrimidines, using base-catalyzed condensation of the formyl group with an activated methylene reagent, followed by substitution at positions 4 and 6 using strongly nucleophilic amines. We report here the molecular and supramolecular structures of two examples, both prepared staring from 2-amino-4,6-dichloropyrimidine-5-carbaldehyde. Ethyl (E)-3-[2-amino-4,6-bis(dimethylamino)pyrimidin-5-yl]-2-cyanoacrylate, (I), was prepared from the starting pyrimidine by successive reaction with ethyl cyanoacetate to give the intermediate (III) (see scheme), followed by excess dimethylamine to give the product (I), while 2-[(2-amino-4,6-di-1-piperidylpyrimidin-5-yl)methylene]malononitrile, (II), was prepared by reaction of the same pyrimidine with malononitrile to form the intermediate (IV) followed by excess piperidine giving the product (II).
In each of compounds (I) (Fig. 1) and (II), which crystallizes with Z' = 2 in the P-1 space group (Fig. 2), the pyrimidine component is markedly nonplanar. The ring-puckering parameters (Cremer & Pople, 1975) (Table 1) are defined for the atom sequence N1—C2—N3—C4—C5—C6 in compound (I) and for Nx1—Cx2—Nx3—Cx4—Cx5—Cx6 (where x = 1 or 2 for the type 1 and type 2 molecules) in compound (II); these parameters show that the conformations of these rings are best described as boat conformations, for which the idealized values of the ring-puckering parameters are θ = 90° and ϕ = (60k)°, where k represent zero or an integer. We have previously observed such nonplanarity in a number of extensively substituted pyrimidine derivatives exhibiting boat (Quesada et al., 2004) or twist-boat (Melguizo et al., 2003; Quesada et al., 2002, 2003) conformations, and by comparison with less extensively substituted analogues, the distortions from planarity were ascribed to steric factors (Melguizo et al., 2003). The occurrence of nonplanar pyrimidine rings here in the presence of four substituents on each pyrimidine ring is certainly consistent with the earlier interpretation. The conformations of the three independent pyrimidine rings in compounds (I) and (II) are thus very similar and the molecules are all chiral: the ring-puckering parameters show that the two independent molecules selected as the asymmetric unit in compound (II) are of the same hand. The piperidine rings in compound (II) all adopt chair conformations. The bond distances are all within the normal ranges and there is no geometric evidence for any significant polarization of the molecular–electronic structures.
The molecules of compound (I) are linked into chains of edge-fused rings by two independent hydrogen bonds (Table 2). Amino atom N2 in the molecule at (x, y, z) acts as hydrogen-bond donor, via H2A and H2B, respectively, to ring atom N1 in the molecule at (2 - x, 1 - y, 1 - z) and carbonyl atom O55 in the molecule at (x, y, 1 + z). Propagation of these two hydrogen bonds by inversion and translation then generates a chain of edge-fused rings running parallel to the [001] direction, with R22(8) (Bernstein et al., 1995) rings centred at (1, 1/2, n + 1/2) (where n represents zero or an integer), and R44(20) rings centred at (1, 1/2, n) (where n represents zero or an integer) (Fig. 3). There are no direction-specific interactions between adjacent chains.
The supramolecular aggregation of compound (II) is dominated by four independent N—H···N hydrogen bonds (Table 3) which link the molecules into a chain of rings somewhat different from that in compound (I), while weaker C—H···N hydrogen bonds link these chains into sheets. The two independent molecules within the selected asymmetric unit are linked by the N—H···N hydrogen bonds involving H12A and H22A to form a dimeric unit which has approximate twofold rotational symmetry around (~0.25, y, ~0.5). These dimeric units are linked by two further N—H···N hydrogen bonds, involving H12B and H22B, into a chain of edge-fused rings running parallel to the [100] direction and containing alternating R22(8) and R22(20) rings (Fig. 4). Fairly weak C—H···N hydrogen bonds, all involving C atoms within the piperidine rings as the donors and nitrile N atoms as the acceptors, link the [100] chains into sheets parallel to (010): the C—H bonds involved are likely to be of low acidity so that the structural significance of these interactions may be marginal.