issue contents
June 1995 issue
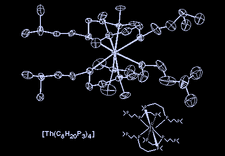
Cover illustration: Dimorphism in Actinide Phosphides: Tetrakis[bis(2-dimethylphosphinoethyl)phosphido]thorium(IV), see Coles, Edwards, Hursthouse & Read, pages 1060--1063. Displacement ellipsoids are plotted at the 50% probability level.
inorganic compounds
Download citation


Download citation


The structures of the two polymorphic forms of K2Ce(NO3)6 are built from discrete irregular [Ce(NO3)6]2− icosahedra. The K atoms ensure the continuity of the structures by ionic contacts.
Download citation


Download citation


The structure of K3Ce2(NO3)9 consists of a three-dimensional network of irregular [Ce2(NO3)9]3− icosahedra with K atoms located in the cavities.
Download citation


Download citation


The triclinic structure of Ba(ReO4)2.H2O closely resembles the pseudomonoclinic space group P2/c pattern with one formula in the asymmetric unit. The BaO10 polyhedron is made up from eight O atoms pertaining to isolated ReO4 tetrahedra and two O atoms from water molecules.
Download citation


Download citation


The structure of Na2O2.8H2O is built up from staggered chains of edge-sharing Na(H2O)6 octahedra. The chains are linked by the peroxide ions via hydrogen bonds.
Download citation


Download citation


HgSeO3 is isomorphous with a series of perovskite-like MSeO3 compounds, and has a structure consisting of a three-dimensional network of corner-sharing HgO6 octahedra with Se4+ ions located in the cavities.
Download citation


Download citation


The O atoms in CuFe2(OH)2(AsO4)2 are cubic close packed; between successive layers only alternate octahedral or tetrahedral sites are occupied by Cu[4+2] or Fe[6] and As atoms. Neighbouring sheets of octahedra are linked by bifurcated hydrogen bonds.
Download citation


Download citation


Hexaammineruthenium(III) trichloride forms monoclinic crystals containing four inequivalent Ru(NH3)6 fragments. The structure is consistent with the ESR spectrum of the compound.
Download citation


Download citation


The structure of NiP4O11 is built from layers of cations and layers of corner-sharing PO4 tetrahedra. Within the layers, rings containing 6 or 14 PO4 tetrahedra are observed.
Download citation


Download citation


The refinement of the structure of phosphorus pentoxide at 233 K results in P—O bond lengths and P—O—P bond angles which are in good agreement with those found in other crystalline phosphates. This is in contrast with the results of an earlier study of this compound.
metal-organic compounds
Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


organic compounds
Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation


Download citation




 journal menu
journal menu