issue contents
July 1998 issue
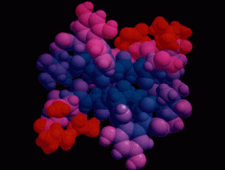
Cover illustration: The balhimycin dimer, with citrate and acetate ions in the binding pockets, colour coded on a scale where the most mobile atoms are red and the least mobile are blue. The binding pockets are clearly the most mobile, consistent with the idea that they can open and close to complex with cell-wall binding peptides. Courtesy of George Sheldrick.
research papers
X-ray diffraction data for the enzyme bovine liver rhodanese have been recorded at 100 K and the crystal structure has been refined anisotropically at 1.36 Å resolution. Details of the crystal structure and of the active site are described.
Efficient algorithms are described for data scaling, map interpolation, automatic averaging mask calculation and averaging operator refinement, for use in density-modification calculations and elsewhere. These algorithms have contributed significantly to the speed and automation achieved in the dm software package.
Two crystal forms of D-monellin, the enantiomer of a natural sweet protein monellin, have been studied by X-ray crystallography. One crystal form (crystal form II), which D-monellin greatly favors in crystallization, shows a completely different dimer contact to all L-monellin crystals reported to date.
Structure of d(CACGCG) d(CGCGTG) in Crystals Grown in the Presence of RutheniumIII Hexammine Chloride
d(CGCGTG) in Crystals Grown in the Presence of RutheniumIII Hexammine Chloride
The structure at 1.64 Å resolution is a left-handed Z-DNA helix. It suggests a non-specific role for the metal ion in helix stabilization.
The structure has been determined by molecular replacement and compared with the acidic isoform.
The X-ray crystal structures of the triclinic form of hen egg-white lysozyme refined against data extending to 0.92 Å at 120 K and 0.95 Å at 295 K are described. The data allowed occupancy refinement of solvent sites.
Estimates of the expected ratio of the free R factor to the standard R factor are calculated for the cases of restrained and unrestrained refinement. Comparison of this Rfree ratio can be used to detect systematic model or weighting errors.
The structure of holo-glyceraldehyde-3-phosphate dehydrogenase from South China Sea lobster reveals subunit similarity and some differences from the thermophilic enzymes.
The crystal structure of r(CGUAC)dG with two independent duplexes in the asymmetric unit has been determined at high resolution. The 2′-hydroxyl groups are engaged in water-mediated hydrogen bonding to all three groups of the nucleotide, i.e.., base, sugar and phosphate.
G·(G·C) base triplets formed by 5′-G and G-3′ binding with the B-DNA octamer duplex d(GCAATTGC)2 are described.
In the crystal structure of L-Tyr-L-Tyr-L-Leu monohydrate, a short intramolecular contact is formed between a Tyr side chain and the peptide NH of the next residue. Reasons are given why this contact represents a weak hydrogen bond.
Crystals of the free soybean trypsin inhibitor have been obtained and the structure determined by molecular replacement. The structure allows a comparison to be made of the reactive-site loop in the complexed and uncomplexed forms and gives an insight into the possible interactions between the homologous Erythrina trypsin inhibitor and tissue-type plasminogen activator.
Human heparin binding protein crystals diffract to atomic resolution when flash-frozen at 120 K. The fully refined 1.1 Å structure is described.
Experiments carried out at EMBL synchrotron beamlines at DESY show there is an advantage in using wavelengths longer than 1 Å for X-ray data collection from small frozen protein crystals.
The projected aquaporin structure is determined in projection to 6 Å resolution by direct methods. Helix centres are located but helix tilt is not evident in the potential maps.
Macromolecular Crystal Annealing: Overcoming Increased Mosaicity Associated with Cryocrystallography
Macromolecular crystal annealing is a simple process that can offset increased mosaicity caused by flash-cooling. The process offers many benefits, such as the recovery of mishandled crystals, and extends the application of cryocrystallography.
The structure of rusticyanin has been determined at 2.1 Å resolution by combining direct methods with the single wavelength anomalous scattering of copper. The results provide clear demonstration of the power of the method in solving a de novo protein structure.
crystallization papers
The single-chain ribosome-inactivating protein saporin isoform 6 isolated from the seeds of S. officinalis has been crystallized under high-salt conditions. The crystals belong to the tetragonal system, space group P4122 or P4322 and diffract to 2.0 Å.
Retinal dehydrogenase type II was purified from an E. coli overexpression system and crystallized in two separate conditions. Two crystal forms were obtained. Only the crystals belonging to space group P212121, grown in conditions which minimize O2 exposure, produced high quality X-ray diffraction data.
With the application of synchrotron radiation, a diffraction resolution of 3.3 Å has been obtained from crystals of human complement component C5.
Phytase from E. coli has been crystallized using a bulk crystallization method without, a conventional precipitant. The enzyme is a 6-phytase involved in the hydrolysis of phytic acid.
The pore-forming outer membrane protein (porin) Omp32 was crystallized in three different crystal forms of space group R3. Two forms were obtained with native porin, the third form occurred with Omp32 containing succinylated lysine residues.
Crystals of this enzyme have been produced and X-ray data collected to 2.8 Å. The space group, unit-cell dimensions and Matthews coefficient have been determined.
The crystallization of a flavoprotein with indole oxygenase activity is described.
Crystals of S. cerevisiae cytoplasmic aspartate aminotransferase that diffract to spacings of 2 Å have been obtained in the presence of pyridoxal phosphate and maleic acid.
Allophycocyanin from red alga P. yezoensis has been crystallized in three crystal forms.
The crystallization and preliminary X-ray analysis of S. campanulata agglutinin complexed with a trisaccharide is reported. The complex crystallizes in space group C2 with six subunits per asymmetric unit and has been solved by molecular replacement at 2.5 Å resolution.
Wild-type and several mutant forms of ribulose-1,5-bisphosphate carboxylase/oxygenase from the green alga, C. reinhardtii, have been crystallized. It is anticipated that the solution of these related structures will reveal the subtle interactions that influence the specificity of the enzyme for CO2 and O2.
GAPDH from S. solfataricus has been crystallized. The crystals diffract to 2.4 Å and belong to the space group P41212 or P43212 with unit-cell parameters a = b = 101.57, c = 179.81 Å.
Crystals of PETN reductase belong to space group P212121 and diffract beyond 1.8 Å. Molecular replacement calculations using Old Yellow enzyme as a search model give a unique solution.
5-Keto-4-deoxyuronate isomerase from E. coli has been crystallized from ammonium sulfate and a suitable cryoprotectant has been identified.
Histidine ammonium-lyase from P. putida was expressed in E. coli, purified to homogeneity, and crystallized by the vapour-diffusion method using polyethylene glycol 3350 as the precipitant. The crystals, which diffract to at least 2.5 Å resolution, exhibit the symmetry of space group P212121, with unit-cell parameters a = 89.7, b = 138.2 and c = 164.8 Å.
The GDP-L-fucose producing enzyme has been crystallized in a trigonal form suitable for high-resolution studies.
Crystals of a barley β-glucan exohydrolase diffract to at least 2.2 Å resolution. The crystals and their heavy-metal derivatives should allow the first structure of a family 3 glycosyl hydrolase to be solved.
short communications
Crystals of both native and a selenomethionine-substituted form of the signal receiver domain of ETR1, an ethylene receptor from A. thaliana, have been obtained and shown to diffract to 2.1 and 2.5 Å, respectively.
This paper reports the crystallization and characterization of a C2-like domain from PKC-δ. The production of selenomethionine-substituted material is described and will assist the solution of the phase problem.
Diffraction-quality crystals have been obtained of the integral membrane protein ferric enterobactin receptor from the outer membrane of E. coli using the zwitterionic detergent lauryldimethylamine oxide, the precipitants PEG 1000 and NaCl, and the additive heptane-1,2,3-triol. The crystals are in orthorhombic space group C2221 with a = 112.2, b = 137.2 and c = 135.4 Å and diffract to 2.5 Å resolution.
fast communications
Type III antifreeze protein was crystallized at 277 K. The resulting structure is essentially identical to the room-temperature structure, thereby supporting the biological relevance of the latter structure.
Crystallization experiments of different proteins were carried out in the presence of a magnetic field. Crystals are found oriented in the field.
addenda and errata
Free 

book reviews
Free 

Free 



 journal menu
journal menu