issue contents
February 2000 issue
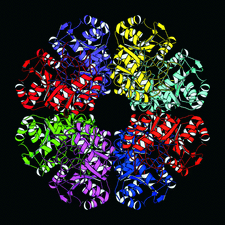
Cover illustration: Yeast porphobilinogen synthase octamer (p. 115).
topical reviews
The currently available crystal structures and sequences of the porphobilinogen synthase family of metalloenzymes are analyzed to evaluate the variation in metal-ion usage by this essential enzyme.
research papers
The structure of xylose isomerase from S. diastaticus No. 7 strain M1033 has been refined at 1.86 Å resolution.
PDB reference: SDXyI, 1qt1
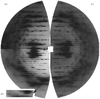
A molecular model of the higher temperature form of the Pf1 protein capsid has been refined against 3 Å resolution X-ray fibre diffraction data using simulated annealing. The resulting model is compared with the model of the lower temperature form.
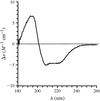
The structure of toxin II of the scorpion A. australis Hector has been refined at 0.96 Å resolution using data collected at room temperature. Most of the solvent molecules were located. A refinement with a multipolar electron-density model (aspherical electron-scattering factors) led to the observation of valence electrons.
A mass-spectrometry based heavy-atom derivative screening method is introduced here as an alternative to the conventional crystal soaking method in identifying suitable phasing derivatives for the crystals of FcγRIII.
A molecular-replacement method is described which simultaneously determines the rotational and translational parameters of all copies of a search model in the crystallographic asymmetric unit of a target structure.
Human cytomegalovirus protease and its inhibitor complex were crystallized and then treated to improve diffraction.
crystallization papers
Single crystals of the intracellular xylanase IXT6 from the thermophilic bacterium B. stearothermophilus T-6 have recently been obtained and used for a full crystallographic data measurement at 2.5 Å resolution.
Crystals of luffaculin, a ribosome-inactivating protein from sponge gourd seeds, have been obtained. The crystals belong to space group C2 and diffract to 2.0 Å resolution.
The membrane-binding core domain of protein 4.1 from human erythrocytes has been crystallized. The methods of crystallization and preliminary X-ray diffraction data are reported.
S100A12, a member of the calgranulin family, isolated from human blood, has been crystallized by vapour diffusion in the presence of Ca2+.
The catalytic subunit of potato ADP-glucose pyrophosphorylase has been crystallized with one crystal form diffracting to 2.8 Å belonging to a space group P2. Another crystal form obtained in the presence of the substrate analog Cr-ATP, belongs to space group 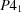 and diffracted to 2.2 Å.
and diffracted to 2.2 Å.
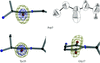
Prolyl-tRNA synthetase from T. thermophilus (ProRSTT) was purified to homogeneity using a five-step purification procedure and was crystallized using ethylene glycol as a precipitant.
The complex between T. thermophilus prolyl-tRNA synthetase (ProRSTT) and its cognate tRNA has been crystallized using two different isoacceptors of tRNAPro.
A recombinant α-amylase from B. stearothermophilus was found to be produced as several isoforms arising from different N-terminal processing. Some of those isoforms were purified to homogeneity and crystallized at 293 K using the hanging-drop vapour-diffusion method.
Recombinant amylosucrase from N. polysaccharea was crystallized by the vapour-diffusion procedure in the presence of polyethylene glycol 6000.
The enzyme phosphonoacetaldehyde hydrolase, which catalyzes the hydrolysis of the unusual C—P bond, has been crystallized from PEG 4000 in space group C2. Identification of NCS in the native Patterson map and its use in initial phasing is described.
The β-carbonic anhydrase from the red alga P. purpureum was expressed in E. coli and purified. Crystals suitable for X-ray structure determination were obtained.
The PDZ domain of Dishevelled protein has been expressed in E. coli, purified and crystallized. The crystals diffract to 2.3 Å resolution and belong to the hexagonal space group P6122 or P6522.
Nitrite reductase from D. desulfuricans ATCC 27774 has been crystallized by the vapour-diffusion method using PEG and CaCl2 as precipitants in the presence of 3-(decylmethylammonium)propane-1-sulfonate. Data collection under cryogenic conditions allowed the determination of the space group P212121, with unit-cell parameters a = 78.94, b = 104.59, c = 143.18 Å.
X-ray studies of recombinant anti-testosterone Fab fragments: the use of PEG 3350 in crystallization
Recombinant anti-testosterone wild-type Fab fragment and mutant Fab fragments with high binding selectivity developed by protein engineering have been crystallized with and without ligands.
The anabolism of L-rhamnose in many pathogenic bacteria presents itself as a possible novel therapeutic target. RmlB is the second of the four enzymes involved in this pathway and work leading to the solving of the structure of this enzyme by molecular replacement is described.
Crystals of M. tuberculosis thymidylate kinase have been obtained in the presence of dTMP, with one molecule per asymmetric unit. They diffract to 1.94 Å resolution using synchrotron radiation.
Orthorhombic crystals that diffract past 2 Å resolution using synchrotron radiation have been obtained for the yeast Ran-binding potein Mog1p.
short communications
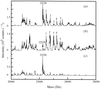
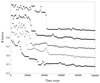
Protein structures can be phased using the anomalous signal of bromide or iodide ions incorporated into the ordered solvent region of crystals by short cryo-soaking. This approach has been tested on various proteins using MAD, SAD or SIRAS methods.
The Shake-and-Bake procedure has been applied to three small proteins that crystallized in space group P1. All trial structures can be guaranteed to converge to solution under a proper choice of conditions.
The structure of human erythrocyte catalase was found to be similar to bovine liver catalase in the orthorhombic crystal form but was not found to contain NADPH.
PDB reference: human erythrocyte catalase, 1qqw
Until now, no aspartic proteinase has been subjected to a successful neutron diffraction analysis, owing to the limited size of the crystals. However, the recent development of the neutron Laue technique at ILL (Grenoble) has allowed the collection of data to 2.2 Å on a complex of endothiapepsin with a transition-state analogue.


 journal menu
journal menu