issue contents
April 2000 issue
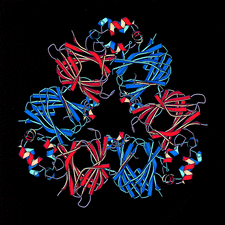
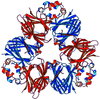
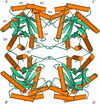
Cover illustration: Threefold-related subunits create a canavalin molecule of pseudo-32 symmetry as a consequence of structural domain redundancy within the polypeptide (p. 411).
research papers
Structures have been determined of the complexes of HIV protease inhibitors L-756,423 with HIV-1 wild-type protease, and of Indinavir and Saquinavir each with the mutant protease containing nine point mutations. Comparative analysis of these structures reveals an alternate binding pocket for the P1–P3 group of Indinavir and L-756,423.
The 1.6 Å structure of the complex between acetohydroxyacid isomeroreductase and its reaction product, dihydroxymethylvalerate, is presented.
PDB reference: Isomeroreductase–dihydroxymethylvalerate complex, 1qmg
The bacteriophage PP7 was crystallized in three different forms, all of space group P1 with two icosahedral particles per cell. The structure was solved to 3.7 Å resolution using one of these crystal forms.
PDB reference: bacteriophage PP7, 1dwn
The Ser195Ala mutant of α-thrombin has been shown to have residual catalytic activity by hydrolyzing fibrinopeptide A(7–22) at Arg16-Gly17.
PDB reference: FPA(7–16)–mutant thrombin, 1dm4
The structure of the seed protein canavalin has been refined to approximately 2.0 Å in cubic and hexagonal unit cells. The structure of the protein has been compared with homologous molecules.
In a previous issue of Acta Crystallographica Section D: Biological Crystallography Eileen Jaffe reviewed the structure and function of porphobilinogen synthases. In this paper we describe the MAD analyses which were undertaken to solve the first structure of an enzyme in this family and how these experiments revealed much about their metal binding properties.
Two examples are described of the use of single-wavelength anomalous dispersion measurements in crystallography. The examples outline the various applications of the anomalous signal, depending on whether it is strong or weak.
In the first part of this series, estimates of the expected value of the ratio of the free R factor to the standard R factor at the convergence of the structure refinement were given. Here, estimates of the variance of this ratio are given and are compared with the observed deviations from the expected values for a selection of refined structures. It is discussed how errors in the functional form of the structure-factor model as well as other types of errors might influence this ratio.
An algebraic analysis leads to bulk-solvent-corrected forms of the Sayre equation and tangent formula, which are verified by empirical numerical calculations for two protein structures with molecular weights of about 100 and 150 kDa.
crystallization papers
Cephalosporin C acylase from Pseudomonas sp. strain N176 has been crystallized. The crystals diffracted to 2.7 Å and belong to space group P41212 or P43212.
Crystals of a truncated but fully active form of S. agalactiae hyaluronate lyase have been obtained by the vapor-diffusion method. The results of the preliminary X-ray diffraction are discussed.
Crystals of alkaline xylanase (xylanase J) from alkaliphilic Bacillus sp. 41M-1 were grown by decreasing the temperature of the protein solution.
Crystallization, data collection to 1.4 Å and determination of the structure of a 54 kDa phospholipase D from Streptomyces sp. strain PMF using multiwavelength anomalous dispersion from a single tungstate ion is reported.
FadR has been crystallized and subjected to initial diffraction studies. Two crystal forms were obtained, both diffracting to 3.5 Å on a rotating-anode generator.
Native and selenomethionyl recombinant, yeast orotidine 5′-phosphate decarboxylase yielded crystals that diffract to less than 2.2 Å. ODCase cocrystals with 6-hydroxyuridine 5′-monophosphate, a postulated transition-state analog, diffract to less than 2.5 Å.
Crystals have been obtained of the VMA13p subunit of the vacuolar V-ATPase of S. cerevisiae. This subunit is essential for catalysis but not for the assembly or targeting of the enzyme.
A variety of strategies, including the use of modified and recombinant forms of the enzyme, have been used to obtain suitable crystals of the human bile-salt dependent lipase.
The aromatic monooxygenase ActVA-Orf6 from S. coelicolor A3(2), which catalyses an unusual oxidation on the actinorhodin biosynthetic pathway, has been crystallized.
The genes encoding the tRNA-modifying enzyme S-adenosylmethionine:tRNA ribosyl transferase/isomerase (QueA) from 12 eubacterial sources were overexpressed in E. coli and the resulting products were purified to homogeneity and subjected to crystallization trials. Crystals suitable for X-ray diffraction experiments were obtained only from the queA gene product of B. subtilis.
Preliminary crystal data have been obtained for the first time for nicotinamide nucleotide transhydrogenase. A 2.1 Å resolution native data set has been collected with an Rmerge of 6.8%.
Three different crystal forms of complexes between arginyl-tRNA synthetase from yeast S. cerevisae (yArgRS) and the yeast second major tRNAArg (tRNA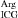 ) isoacceptor have been crystallized by the hanging-drop vapour-diffusion method, one of which diffracts to 2.2 Å resolution. Crystal structures have been solved by the molecular-replacement method.
) isoacceptor have been crystallized by the hanging-drop vapour-diffusion method, one of which diffracts to 2.2 Å resolution. Crystal structures have been solved by the molecular-replacement method.
The crystallization and preliminary X-ray analysis of the haemoglobin I from the fish L. anisitsi is reported.
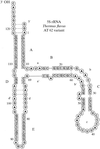
T. flavus 5S rRNA, with a molecular weight of about 40 kDa, was modified at the 5′ and 3′ ends and crystals were obtained under earth and microgravity conditions. A complete data set to 7.8 Å resolution was obtained.
Squid neuronal Sec1 is an evolutionary conserved protein involved in the regulation of neurotransmission. Crystals of space group P3121 diffracting to 3.3 Å resolution were obtained from a His-tagged construct.
Crystals of human nucleoside diphosphate kinase A have been obtained in space group P21 and X-ray diffraction data have been collected to 2.15 Å resolution.
A tryptophan-containing variant of the wild-type B1 domain of protein L and two mutants, Lys61Ala and Val49Ala, have been crystallized. Full data sets have been collected for the wild type and the Lys61Ala and Val49Ala mutants to resolutions of 1.7, 2.3 and 1.8 Å, respectively.
E. coli L-asparaginase II with a Ser58Ala mutation has been crystallized in a new orthorhombic form (space group P21212) with six protein molecules in the asymmetric unit.
Shikimate dehydrogenase from E. coli has been crystallized by the vapour-diffusion method using ammonium sulfate as a precipitant. Mass spectrometry confirmed the purity of the enzyme and dynamic light scattering was used to find the appropriate additives to yield a monodisperse enzyme solution.
The structure of KDO8P synthase from both monoclinic and cubic crystals confirms that the enzyme is a tetramer and not a trimer as suggested by earlier biochemical studies.
short communications
A 1.1 Å resolution structure of porcine pancreatic elastase is provided with a description of the hydrogen-bonding geometry in the active site.
PDB reference: porcine pancreatic elastase, 1qnj
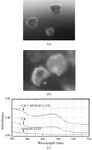
In crystallization trials, recombinant human α-L-iduronidase forms spherulites with X-ray diffraction patterns and Congo red binding reminiscent of the amyloid state.


 journal menu
journal menu