issue contents
March 2002 issue
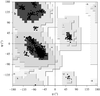
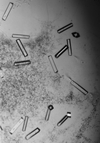
Cover illustration: Different crystal morphologies of urate oxidase (from A. flavus) obtained in different concentrations of various PEGs. The nucleation rate follows the variations of the second virial coefficient (p. 472).
research papers
The crystal structure of the FERM domain of merlin has been solved by molecular replacement and refined to 1.8 Å resolution. Missense mutations causing neurofibromatosis type 2 are examined.
PDB reference: merlin FERM domain, 1h4r, r1h4rsf
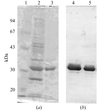
Five small affinity tags had differing effects on the crystallization of P. furiosus maltodextrin-binding protein and the quality of the crystals that were obtained.
The crystal structure of the Y. pestis type III secretion chaperone SycE has been determined at a resolution of 1.95 Å.
PDB reference: SycE, 1k6z, r1k6zsf
The structure of a hexameric form of S100A12, a member of the S100 family of proteins, has been solved at 2.7 Å resolution. The results suggest a functional significance for the hexamer.
PDB reference: S100A12 hexameric form, 1gqm, r1gqmsf
The resolution and the definition of the structure of garlic lectin could be improved by re-refinement using reprocessed data employing different program packages. The re-refined structure, amongst other things, leads to a better identification of residues at the variable locations in the sequence and hence of isolectins.
PDB reference: garlic lectin, 1kj1, r1kj1sf
The crystal structure of apo-AhrC, the hexameric arginine repressor/activator protein from B. subtilis, has been determined. The structure has been compared with previous 30 Å resolution EM studies of the protein and with crystal structures of its homologues from E. coli and B. stearothermophilus.
PDB reference: AhrC, 1f9n, r1f9nsf
MAD phases have been combined with the structure-factor magnitudes in the refinement by REFMAC of a platinated decanucleotide. They contributed significantly to the determination of the structure of the ordered water molecules and of a spermine molecule involved in intermolecular contacts.
NDB reference: platinated decanucleotide duplex, DDJ075
Six structures of bovine pancreatic ribonuclease at six pH values have been refined and analyzed. A comparative analysis of the six models and some biological implications are discussed.
The preparation and crystal structure determination of the active-site Cys25→Ser mutant of human cathepsin S at 2.2 Å resolution is described. This structure clearly defines both the catalytic triad and the substrate-binding region.
PDB reference: cathepsin S, 1glo, r1glosf
The resolution of copper nitrite reductase crystals has been improved to 1 Å through a simple procedure of `in situ' annealing.
X-ray imaging and diffraction illuminate the character and origin of protein crystal disorder created by flash-cooling and how annealing can reduce this disorder. Annealing at fixed temperatures well below 273 K should provide a reliable low-risk approach for reducing cooling damage in more disordered crystals.
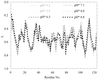
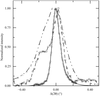
The results from this small-angle scattering study have been extended to the crystallization of large proteins in the presence of polyethylene glycol. The protein crystallization, the nucleation rate and the different morphological crystal shapes obtained were correlated with the second virial coefficient.
The structure of porcine β-lactoglobulin shows a novel and unusual dimerization motif not previously seen in members of the lipocalin family. This partially explains the markedly different physicochemical properties of porcine and bovine β-lactoglobulin, despite these proteins sharing 66% amino-acid identity.
PDB reference: porcine β-lactoglobulin, 1exs, r1exssf
A pattern-recognition search algorithm is described that interprets X-ray electron-density maps and forms the initial stage in automated electron-density map interpretation.
Numerous examples show that with accurate diffraction data SAD phasing leads to a rapid solution of macromolecular crystal structures.
crystallization papers
Rhombohedral crystals of L-aminoacylase from T. litoralis have been grown by the sitting-drop vapour-diffusion technique from recombinant protein expressed in E. coli. The crystals show diffraction to 2.8 Å resolution. The position of the zinc ion has been located using the anomalous data.
Crystallization and preliminary diffraction results for the Oct-1/SNAP190 peptide/DNA complex are presented.
The angiogenesis inhibitor angiostatin has been crystallized and a complete data set to 1.75 Å resolution has been collected. The crystals belong to the P41212 space group, with unit-cell parameters a = b = 56.94, c = 192.97 Å.
The M. pneumoniae HPr kinase/phosphatase (HPrK/P) was crystallized in the space group P212121, with unit-cell parameters a = 117.1, b = 127.7, c = 170.7 Å. Both X-ray and biophysical data indicate that HPrK/P is a functional hexamer that undergoes an ATP-binding-induced conformational change.
RFC boxes II–VIII of replication factor C from M. jannaschii have been crystallized and native data have been collected at 3.2 Å resolution using synchrotron X-rays.
Crystallogenesis and diffraction to a resolution of 2.7 Å are described for the dihaem cytochrome c peroxidase of R. capsulatus, the first example of this type of enzyme isolated from a phototrophic bacterium.
The prokaryotic hydrogenase maturation factor protein HypF (82 kDa) contains a 10 kDa domain displaying structural homology to prokaryotic and eukaryotic acylphosphatases. Two different crystal forms of the HypF N-terminal domain have been characterized.
The diadenosine tetraphosphate hydrolase from C. elegans has been crystallized in the presence of a substrate analogue. The crystals belong to space group P21 and diffract to 2.0 Å resolution.
The preliminary X-ray analysis of recombinant human interleukin-22, a novel member of the cytokine family, is reported.
β-Xylosidase from the thermophilic anaerobe T. saccharolyticum has been crystallized at 296 K using the hanging-drop vapour-diffusion method. The crystal diffracts to 2.4 Å resolution with synchrotron X-rays.
Recombinant EphB2/ephrin-B2 receptor-ligand complex has been purified, biochemically characterized and studied for crystallization conditions. Of the two different cocrystal forms generated, one is suitable for X-ray diffraction studies.
The carbohydrate-recognition domain of p58 was produced in insect cells and in E. coli, purified and crystallized using Li2SO4 as a precipitant. Crystals belong to the space group I222, with unit-cell parameters a = 49.6, b = 86.1, c = 128.1 Å and one molecule per asymmetric unit. These crystals diffract to at least 1.46 Å resolution.
Crystallization and preliminary analysis of the ATPase domain of the ABC transporter HlyB from E. coli are described.
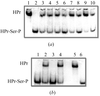
About 40% incorporation of SeMet in Man5A β-mannanase from blue mussel, M. edulis, was obtained with the commercial P. pastoris expression system. The presence of selenium in the crystals was confirmed by an X-ray fluorescence scan. Diffraction to 1.5 Å has been obtained.
Alcohol dehydrogenase from the protozoan parasite E. histolytica has been crystallizaed in space group C2221 with unit-cell parameters a = 76.89, b = 234.24, c = 96.24 Å and a dimer per asymmetric unit. The crystals diffract to 1.9 Å.
Diaminopimelate decarboxylase from E. coli was cloned, expressed, purified and crystallized. Large well ordered crystals diffract to at least 2.1 Å and belong to space group P6122, with unit-cell parameters a = b = 98.6, c = 177 Å.
The crystallization of a chiral PND–DNA duplex is reported. Synchrotron X-ray radiation was used to collect diffraction data to 1.85 Å resolution.
In order to confirm the hypothesis that the previously reported C-terminal fragment of the major pneumococcal autolysin was not the complete choline-binding domain of this enzyme, the full-length C-terminal domain has been crystallized. Diffraction data to 3.2 Å resolution allowed the determination of the space group (P21) and the unit-cell parameters (a = 51.9, b = 30.3, c = 113.6 Å, β = 96.4°).
Ascorbate peroxidase from tobacco plants has been purified and crystallized in the orthorhombic space group P212121. A native data set has been collected to 1.6 Å resolution using the KEK-PF synchrotron-radiation source.
Malonamidase E2, an amidase signature family member, was crystallized and analyzed by X-ray crystallographic methods at a preliminary level.
SEDL with a putative role in ER-to-Golgi transport has been overexpressed, crystallized, and analyzed by X-ray crystallographic method at a preliminary level.
short communications
A new crystal form of the DNA Holliday junction is formed by d(CCGGTACCGG)2 which allows the effects of sequence and lattice type on junction geometry to be distinguished.
NDB reference: UD0015


 journal menu
journal menu