issue contents
July 2005 issue
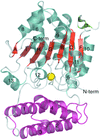
Cover illustration: The protein product of the At2g17340 gene from Arabidopsis thaliana (p. 630).
protein structure communications
Open  access
access
 access
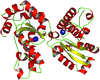
accessThe crystal structures of two manganese superoxide dismutases from B. anthracis were solved by X-ray crystallography using molecular replacement.
Open  access
access
 access
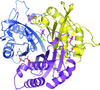
accessA double mutation designed to disrupt binding of isoprenoid diphosphate to an enzyme involved in isoprenoid biosynthesis was made and the structure determined. Despite the removal of six hydrogen-bonding interactions, the ligand, acquired during production in E. coli, remains bound. The reasons for this are discussed.
structural genomics communications
The crystal structure of the 40.8 kDa At2g17340 protein from A. thaliana was determined at 1.7 Å resolution. The structure provides the first insight into the structural organization of the Pfam01937.11 family and establishes that the proteins of this family coordinate a metal in its putative active site.
PDB reference: At2g17340, 1xfi, r1xfisf
The crystal structure of ST1625p, a protein encoded by a hypothetical open reading frame ST1625 in the genome of the hyperthermophilic archaeon Sulfolobus tokodaii, was determined at 2.2 Å resolution. The structure of ST1625p consists of a unique superhelix with a low-level structure resemblance to doamins from other proteins with known three-dimensional structures.
PDB reference: ST1625p, 1wy6, r1wy6sf
The expression, purification, crystallization, and structure determination of NAD-kinase from T. maritima are reported. Similarity to other NAD-kinases as well as homo-oligomrization state of the enzyme from T. maritima are discussed.
PDB reference: NAD kinase, 1yt5, r1yt5sf
The crystal structure of the 18 kDa At3g22680 gene product from A. thaliana was determined at 1.6 Å resolution. At3g22680 shows no structural homology to any other known proteins and represents a new fold in protein conformational space.
PDB reference: At3g22680 gene product, 1vk5, r1vk5sf
crystallization communications
Partial proteolysis of the avian reovirus cell-attachment protein σC yields a major homotrimeric C-terminal fragment that presumably contains the receptor-binding domain. This fragment has been crystallized in the presence and absence of zinc sulfate and cadmium sulfate. One of the crystal forms diffracts synchrotron X-rays to 2.2–2.3 Å.
The use of lipopolysaccharide in the crystallization of the integral membrane protein MsbA was critical to the overall stability of the crystals and led to an increase in diffraction resolution.
A double mutant of yeast nuclear thiol peroxidase has been crystallized in a truncated form. The crystal belongs to space group P32, with unit-cell parameters a = b = 37.54, c = 83.26 Å. A diffraction data set has been collected to 1.8 Å resolution.
The C-terminal domain of adenylyltransferase (ATase) from Escherichia coli has been overexpressed, purified and crystallized in a form suitable for structure analysis.
Human STAT1 (1–683) has been crystallized in the presence of a receptor-derived phosphopeptide. A diffraction data set has been collected and processed to 3.0 Å resolution.
All three components of the toluene dioxygenase system have been expressed, purified and crystallized.
Human recombinant EF-hand Ca2+-binding protein S100B has been purified and crystallized. A complete data set was recorded to 1.9 Å.
The expression, purification and preliminary X-ray diffraction studies of a novel N-acetyl-L-citrulline deacetylase from X. campestris are reported.
Open  access
access
 access
accessAn unannotated protein reported from B. subtilis has been expressed in E. coli and identified as possessing penicillin V acylase activity. The crystallization and preliminary crystallographic analysis of this penicillin V acylase is presented.
The Holliday junction-cutting enzyme Hjc from A. fulgidus was crystallized by the counter-diffusion method in agarose gel and complete data were collected at 2.7 Å resolution using synchrotron radiation.
Orthorhombic crystals of mouse 3(17)α-hydroxysteroid dehydrogenase were obtained from buffered polyethylene glycol solutions. The crystals diffracted to a resolution of 1.8 Å at the Swiss Light Source beamline X06SA.
A conserved hypothetical protein XC1692 from X. campestris pv. campestris has been overexpressed in E. coli. The purified recombinant protein crystallized in a variety of forms and diffracted to a resolution of at least 1.45 Å.
A conserved hypothetical protein XC229 from X. campestris pv. campestris has been overexpressed in E. coli, purified and crystallized. A crystal of the purified recombinant protein diffracted to a resolution of 1.80 Å.
A putative polyketide-synthesis protein XC5357 from X. campestris pv. campestris has been overexpressed in E. coli, purified and crystallized. The crystals diffracted to a resolution of at least 1.85 Å.
A putative ApaG gene product from X. campestris pv. campestris was overexpressed in E. coli, purified and crystallized. The crystals diffracted to a resolution of at least 2.3 Å.
A conserved hypothetical protein XC6422 from X. campestris pv. campestris has been overexpressed in E. coli, purified and crystallized. Crystals obtained from the purified recombinant protein showed a variety of forms that diffracted to at least 1.6 Å resolution.
A putative repressor for the multiple antibiotic resistance operon from a plant pathogen X. campestris pv. campestris has been overexpressed in E. coli, purified and crystallized. The crystals diffracted to 2.3 Å with good quality.
The crystallization of 2-deoxy-scyllo-inosose synthase, the key enzyme in the biosynthesis of 2-deoxystreptamine-containing aminoglycoside antibiotics, is reported.
Recombinant BchU from C. tepidum has been crystallized. Crystals diffract to 2.27 Å using synchrotron radiation at SPring-8 and belong to space group P6122 or P6522, with unit-cell parameters a = b = 81.5, c = 250.7 Å.
A truncated form of HscA (52 kDa) containing both nucleotide- and substrate-binding domains has been crystallized and analyzed by X-ray diffraction. The crystal belongs to space group P212121 and diffracts to 2.9 Å.
M. tuberculosis dihydrodipicolinate reductase, the enzyme that catalyzes the second committed step of lysine biosynthesis, has been cloned, expressed, purified and crystallized in three different crystal forms.


 journal menu
journal menu












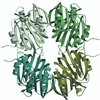













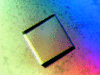








![[publBio]](http://journals.iucr.org/logos/publbio.gif)





