issue contents
May 2006 issue
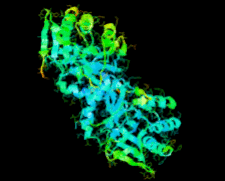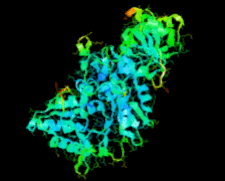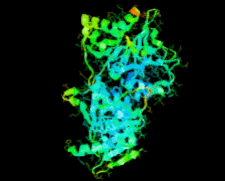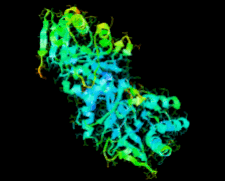
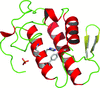
Cover illustration: Ribose 5-phosphate isomerase from Plasmodium falciparum (p. 427).
protein structure communications
The crystal structure of myotoxin II from A. nummifer venom has been determined at 2.08 Å resolution.
PDB reference: myotoxin II, 2aoz, r2aozsf
structural genomics communications
The crystal structure of ribose 5-phosphate isomerase from P. falciparum has been determined at 2.09 Å resolution. The structure is very similar to those of RpiA from other species, but is in some ways more similar to bacterial homologs than to those from higher eukaryotes.
PDB reference: ribose 5-phosphate isomerase, 2f8m, r2f8msf
crystallization communications
Crystals of human PRPP synthetase 1 (PRS1) in complex with Mg2+, Pi and ATP were obtained and diffraction data were collected to 2.6 Å resolution.
Open  access
access
 access
accessCrystals of a selenomethionine-incorporated YphC–GDP complex have been grown using the hanging-drop vapour-diffusion method and polyethylene glycol as a precipitating agent.
The crystallization and preliminary X-ray characterization of the polysaccharide lyase family 11 rhamnogalacturonan lyase are presented.
Open  access
access
 access
accessA method is presented that allowed the diffraction limit of crystals of the secreted chorismate mutase from M. tuberculosis to be improved from approximately 3.5 to 1.3 Å. To obtain large well diffracting crystals, it was critical to initiate crystallization at higher precipitant concentration and then transfer the drops to lower precipitant concentrations within 5–15 min.
XAC1151, a small heat-shock protein from X. axonopodis pv. citri belonging to the α-crystallin family, was crystallized using the sitting-drop vapour-diffusion method in the presence of ammonium phosphate. X-ray diffraction data were collected to 1.65 Å resolution using a synchrotron-radiation source.
Avian adenovirus long-fibre head trimers were expressed, purified and crystallized. The crystals belong to space group C2 (unit-cell parameters a = 216.5, b = 59.2, c = 57.5 Å, β = 101.3°). A complete highly redundant data set was collected to 2.2 Å resolution at 100 K using a rotating-anode X-ray source.
Joint X-ray and neutron crystallographic data have been collected from the oligonucleotide d(CGCGCG) crystallized without polyamine and at low pH in order to study hydration in the protein-binding major groove of Z-DNA.
A complex of the essential splicing factor U2AF65 and a deoxyuridine oligonucleotide has been crystallized by modification of an interdomain linker.
The crystallization and X-ray data analysis of three crystal forms of the outer membrane pyoverdine transducer FpvA from P. aeruginosa bound to ferripyoverdine are described. The resolution of the crystals ranges from 3.15 to 2.7 Å depending on the crystal form; all were obtained in the presence of C8E4 detergent.
Lipoprotein e (P4) is a class C acid phosphatase and a potential vaccine candidate for nontypeable H. influenzae infections. This paper reports the crystallization of recombinant e (P4) and the acquisition of a 1.7 Å resolution native X-ray diffraction data set.
S100 proteins are differentially expressed during epithelial cell maturation, tumorigenesis and inflammation. The novel human S100A15 protein has been cloned, expressed, purified and crystallized in two crystal forms, a triclinic and a monoclinic form, which diffract to 1.7 and 2.0 Å, respectively.
CooA from C. hydrogenoformans has been crystallized by the vapour-diffusion method using polyethylene glycol as a precipitant. The crystal diffracted to 2.3 Å resolution.
The CRD domain of GRP from H. sapiens has been expressed, purified and crystallized and X-ray diffraction data have been collected to a resolution of 2.0 Å.
The crystallogenesis of bacteriophage P22 tail-fiber gp26 is described. To study possible pH-induced conformational changes in gp26 structure, native trimeric gp26 has been crystallized at acidic pH (4.6) and a chimera of gp26 fused to maltose-binding protein (MBP-gp26) has been crystallized at neutral and alkaline pH (7-10).
Crystals of glutamate-1-semialdehyde aminotransferase (GSAT) from B. subtilis were obtained and diffraction data were collected to 2.0 Å resolution.
The crystallization and preliminary X-ray characterization of a family PL-15 exotype alginate lyase are presented.


 journal menu
journal menu


























![[publBio]](http://journals.iucr.org/logos/publbio.gif)





