issue contents
March 2005 issue
Cover illustration: Glutathione S-transferase from Schistosoma japonicum in a new crystal form (p. 263).
protein structure communications
An analysis of the protein–ligand interactions in two crystal structures of DHFR-TS from C. hominis reveals a possible structural basis for observed antifolate resistance in C. hominis DHFR. A comparison with the structure of human DHFR reveals residue substitutions that may be exploited for the design of species-selective inhibitors.
The crystal structure of the 26 kDa glutathione S-transferase from S. japonicum (SjGST) was determined at 3 Å resolution in the new space group P212121. The structure of orthorhombic SjGST reveals unique features of the ligand-binding site and dimer interface when compared with previously reported structures.
PDB reference: glutathione S-transferase, 1y6e, r1y6esf
crystallization communications
Crystallization and X-ray diffraction methods for native A. tumefaciens ADP-glucose pyrophosphorylase and its selenomethionyl derivative are described. Two crystal forms are identified, both of which diffract to 2 Å.
The crystallization of a hypothetical acetyltransferase from the archaeon P. furiosus by hanging-drop vapour diffusion is reported in space group P622.
The dhlIVa gene coding for DehIVa was expressed in Escherichia coli and the protein was purified and crystallized using the hanging-drop method.
A truncated soluble human semicarbazide-sensitive amine oxidase has been crystallized. Data were collected to 2.5 Å from a crystal suffering from twinning, pseudo-symmetry and anisotropy. The structure was solved in space group P43.
Crystals of the aspartate carbamoyltransferase of the psychrophile M. profunda diffract X-rays to 2.85 Å. Three catalytic and three regulatory subunits are predicted per asymmetric unit.
Human spliceosomal protein TXNL4B was purified and crystallized.
Open  access
access
 access
accessA high-affinity mutant CD8 (haCD8) has been developed with the aim of developing a therapeutic immunosuppressor. In order to fully understand the nature of the haCD8 interaction, this protein was crystallized using the sitting-drop vapour-diffusion method.
The crystallization and preliminary characterization of the family PL-7 alginate lyases A1-II and A1-II′ from Sphingomonas sp. A1 are presented.
Reducing-end-xylose releasing exo-oligoxylanase (Rex) from B. halodurans C-125 was crystallized. A diffraction data set was collected to 1.35 Å resolution.
A thermostable ribonuclease HIII from B. stearothermophilus (Bst RNase HIII) was crystallized and preliminary crystallographic studies were performed. Plate-like overlapping polycrystals were grown by the sitting-drop vapour-diffusion method at 283 K.
The mRNA-binding domain of M. thermoacetica selenocysteine-specific elongation factor SelB (residues 512–634, SelB-M) was overproduced in E. coli and its cognate mRNA ligand, 23 nucleotides of the SECIS RNA hairpin, was chemically prepared. The purified SelB-M–SECIS RNA complex has been crystallized in space group P21212 and diffracted to 2.3 Å.
Acetylene hydratase, a tungsten-containing hydroxylase that converts acetylene to acetaldehyde, has been purified and crystallized under anaerobic conditions.
A maltooligosaccharide-metabolizing enzyme from T. vulgaris R-47 (TGA) homologous to glucoamylase degrades maltooligosaccharides more efficiently than starch, unlike fungal glucoamylases. TGA was crystallized and the state of the protein in solution was analyzed by gel-filtration chromatography.
Open  access
access
 access
accessExpression, purification and crystallization of the cell-division protein YgfE from Escherichia coli
An open reading frame from E. coli MG1655 has been cloned, expressed and purified. Crystals obtained from the purified recombinant protein have been obtained in a variety of different forms diffracting to 1.8 Å resolution.
The psychrophilic protease Apa1 consisting of a subtilisin-like region and a large insert (148 residues) with unknown structure has been crystallized. Collection of a data set to 1.78 Å resolution and molecular-replacement searches are reported.
This paper describes the first crystallization study of a flavoprotein monooxygenase that can cleave an aromatic compound without requiring a metal-ion cofactor.
Two outer membrane factor family proteins, OprM and OprN, from a tripartite efflux pump found in P. aeruginosa were crystallized. A diffraction data set was collected to 3.8 Å resolution in the space group C2 for OprM crystals.
A human kynurenine aminotransferase II homologue from P. horikoshii OT3 has been overproduced in E. coli, purified, and characterized. Crystals of this protein have been obtained and analyzed by X-ray diffraction.
The hyperthermostable thioredoxin peroxidase from the aerobic hyperthermophilic archaeon A. pernix K1 was crystallized. The crystal diffracted to 2.7 Å resolution.
The first crystal structure of a Mimosoideae lectin, Parkia platycephala has been solved by MAD phasing using 5-bromo-4-chloro-3-indolyl-α-D-mannose as an anomalous X-ray scatterer. This strategy may be useful for structure elucidation of novel lectins or when molecular replacement methods fail.
Crystallization and preliminary crystallographic analysis of the site-specific DNA nickase Nb.BspD6I
The site-specific DNA nickase Nb.BspD6I has been crystallized in space group P21, with unit-cell parameters a = 57.76, b = 90.67, c = 71.71, β = 110.1°.
A protein disulfide oxidoreductase from A. pernix K1 has been crystallized for the first time. The crystals belong to space group I222 or I212121 and diffract to 1.93 Å resolution.


 journal menu
journal menu











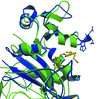



















![[publBio]](http://journals.iucr.org/logos/publbio.gif)





