issue contents
December 2005 issue
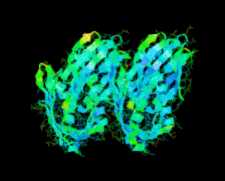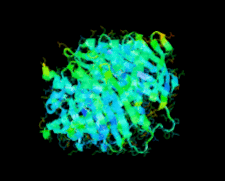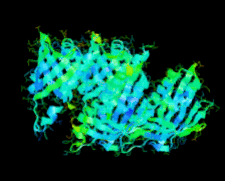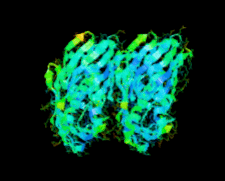
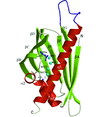
Cover illustration: A conserved hypothetical protein, TTHA0849 from Thermus thermophilus HB8 (p. 1027).
structural genomics communications
The crystal structure of a conserved hypothetical protein, TTHA0849 from T. thermophilus HB8, has been determined at 2.4 Å resolution. The compact α+β structure shows the typical folding of the steroidogenic acute regulatory-related lipid-transfer (START) domain.
PDB reference: TTHA0849, 2d4r, r2d4rsf
crystallization communications
The expression, purification and crystallization of 17β-hydroxysteroid dehydrogenase from the filamentous fungus C. lunatus and its Y167F mutant, both in the apo form, are described. X-ray diffraction analysis and phasing by Patterson-search techniques are reported.
It is shown how protein crystallization results can be used to identify buffers that improve protein solubility and, in turn, crystallization success.
Crystallization of the α-glucosidase MalA from S. solfataricus belonging to glycoside hydrolase family 31.
The two C-terminal domains of the cellulase ctCel9D-Cel44A from C. thermocellum cellulosome have been crystallized in tetragonal space group P43212 and X-ray diffraction data have been collected to 2.1 and 2.8 Å from native and seleno-L-methionine-derivative crystals, respectively.
P. gingivalis prolyl tripeptidyl aminopeptidase has been crystallized by the vapour-diffusion method. Diffraction data have been collected and processed to 2.1 Å resolution.
Well diffracting decamer crystals of the hepatitis delta virus RNA-editing site were prepared, but exhibited merohedral twinning and base averaging owing to duplex symmetry. A longer asymmetric construct that includes additional flanking RNA sequences has been crystallized that does not appear to exhibit these defects.
The crystallization and preliminary X-ray analysis of a β-D-xylosidase from G. stearothermophilus T-6, a family 43 glycoside hydrolase, is described. Native and catalytic inactive mutants of the enzymes were crystallized in two different space groups, orthorhombic P21212 and tetragonal P41212 (or the enantiomorphic space group P43212), using a sensitive cryoprotocol. The latter crystal form diffracted X-rays to a resolution of 2.2 Å.
Crystals of OsAGPR were obtained using the sitting-drop vapour-diffusion method at 293 K and diffract X-rays to at least 1.8 Å resolution. They belong to the hexagonal space group P61, with unit-cell parameters a = 86.11, c = 316.3 Å.
Crystals of the 130 kDa E. coli Mfd protein have been grown and analysed by X-ray diffraction techniques to 3.2 Å resolution. Phases were obtained by single-wavelength anomalous dispersion from selenomethionyl-substituted crystals.
Optimization of crystallization conditions and cryoprotectants decreased the anisotropy of the diffraction obtained from 3B5H10 Fab crystals. Dehydration improved the resolution of cryoprotected 3B5H10 crystals from 2.6 to 1.9 Å, but changed the space group of the crystals from P21212 to P21.
Alanine and glutamine mutations were made to the same 15 lysine positions on the surface of E. coli malate synthase G and the impact on crystallization observed. The results support lysine replacement for improvement of crystallization and provide insight into site selection and type of amino-acid replacement.
The crystallization and preliminary X-ray diffraction analysis of the L-fuculose-1-phosphate aldolase (FucA) from T. thermophilus HB8. Native diffraction data set was collected to a resolution of 1.9 Å.
The 31.3 kDa Ap4A hydrolase from Shigella flexneri 2a has been cloned, expressed and purified using an Escherichia coli expression system. Crystals of Ap4A hydrolase have been obtained by the hanging-drop technique at 291 K using PEG 550 MME as precipitant.
A lipoprotein implicated in mycobacterial cell-wall biosynthesis, LpqW, was expressed in E. coli. Crystals were obtained that diffracted to 2.4 Å resolution.
The first crystallographic study of an isolated WW domain is reported. Single crystals of the WW4 domain of the Nedd4-2 ubiquitin–protein ligase contain a high solvent content of 74% and diffract X-rays to 2.5 Å resolution.
Crystallization of Arabidopsis thaliana cyclophilin 38. The crystal diffracts X-rays to 2.5 Å resolution.
Mouse MHC class I H-2Db in complex with human β2m and the LCMV-derived peptide gp33 has been produced and crystallized. Resolution of the structure of this complex combined with the structural comparison with the previously solved crystal structure of H-2Db/mβ2m/gp33 should lead to a better understanding of how the β2m subunit affects the overall conformation of MHC complexes as well as the stability of the presented peptides.
Open  access
access
 access
accessRecombinant human CLEC-2 was crystallized in the orthorhombic space group P212121 and X-ray diffraction data were collected to 2.0 Å.
The crystallization of HLA-B*2706 in complex with two peptides is reported.
Crystals of the archaeal DNA ligase from Pyrococcus furiosus were obtained using 6.6%(v/v) ethanol as a precipitant and diffracted X-rays to 1.7 Å resolution.


 journal menu
journal menu




























![[publBio]](http://journals.iucr.org/logos/publbio.gif)





