issue contents
October 2006 issue
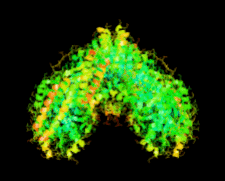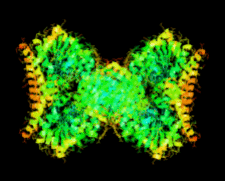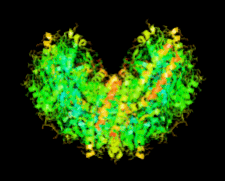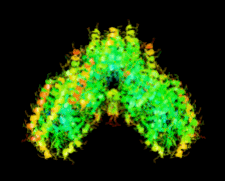
Cover illustration: The first structure of a microbial aspartokinase, that from Methanococcus jannaschii, determined in the presence of the amino-acid substrate L-aspartic acid and the nucleotide product MgADP (p. 962).
protein structure communications
Open  access
access
 access
accessThe structure of the heterotrimeric PCNA complex from S. sulfataricus is reported to 2.3 Å.
PDB reference: PCNA, 2ix2, r2ix2sf
Open  access
access
 access
accessThe crystal structure of S. aureus guanylate monophosphate kinase has been determined to 1.9 Å resolution, revealing both open and closed forms within the asymmetric unit. These structures may be of use in anti-bacterial drug design.
PDB reference: guanylate monophosphate kinase, 2j41, r2j41sf
Open  access
access
 access
accessThe crystallization and preliminary X-ray structure at 1.9 Å resolution of the fungal laccase from C. maxima are presented.
PDB reference: laccase, 2h5u, r2h5usf
The X-ray structure of the tetragonal form of apo acyl-CoA-binding protein (ACBP) from the Harderian gland of the South American armadillo Chaetophractus villosus has been solved.
PDB reference: armadillo ACBP, 2fdq, r2fdqsf
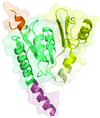
The first structure of a microbial aspartokinase reveals details of its quaternary structure and the mode of substrate binding and provides insights into the catalytic mechanism.
PDB reference: aspartokinase, 2hmf, r2hmfsf
crystallization communications
The crystallization and preliminary X-ray crystallographic analysis of protein YtlP from B. subtilis is reported.
Sample preparation, crystallization and preliminary X-ray analysis are reported for two B. pertussis extracytoplasmic solute receptors.
Crystals of an N-terminally truncated variant of the Salmonella flagellar ATPase FliI, which exports substrate proteins into the central channel of the growing flagellar structure by utilizing the energy of ATP hydrolysis, have been obtained and characterized by X-ray diffraction.
LmACR2 from L. major is the first rhodanese-like enzyme directly involved in the reduction of arsenate and antimonate to be crystallized. Diffraction data have been collected to 1.99 Å resolution using synchrotron X-rays.
Acetylornithine aminotransferases, members of the type I subgroup II family of PLP-dependent enzymes, from S. typhimurium and E. coli have been cloned, overexpressed, purified and crystallized.
Methionine synthase (MetE) from S. mutans was expressed, purified and crystallized. Diffraction data have been collected to 2.2 Å resolution.
Two enzymes responsible for arginine biosynthesis in M. tuberculosis were expressed in Escherichia coli, then purified to homogeneity. Preliminary X-ray analysis of diffraction-quality crystals grown from each enzyme are reported.
PhzS, an FAD-dependent monooxygenase that catalyzes a reaction involved in the biosynthesis of the virulence factor pyocyanin in P. aeruginosa, was cloned, overexpressed and crystallized. Data collection from native and seleno-L-methionine-labelled crystals is reported.
A hyperthermophilic archaeal Rieske iron–sulfur protein (sulredoxin) variant, SDX-triple (H44I/A45C/H64C), having a rationally designed rubredoxin-like mononuclear iron site in place of a Rieske [2Fe–2S] centre, has been crystallized. The P1 crystals of the SDX-triple variant diffract to 1.63 Å resolution using synchrotron radiation.
Mouse carnosinase was crystallized in complex with Zn2+ or Mn2+ and the complexes are undergoing structure determination by the MAD method.
A CN-hydrolase superfamily protein from the plant pathogen X. campestris has been overexpressed in E. coli, purified and crystallized.
CTP:phosphoethanolamine cytidylyltransferase from S. cerevisiae has been expressed, purified and crystallized.
Open  access
access
 access
accessA putative pyridoxal kinase from B. subtilis has been cloned, overexpressed, purified and crystallized and data have been collected to 2.8 Å resolution.
Open  access
access
 access
accessThe gene encoding HpcG from the homoprotocatechuate (4-hydroxyphenylacetic acid) degradative pathway of E. coli C has been cloned and expressed and the protein has been purified. Crystals obtained from the purified recombinant enzyme, belonging to a tetragonal space group, diffracted to a resolution of 2.1 Å.
UvsY, the recombination mediator protein of bacteriophage T4, has been crystallized in both native and selenium-substituted forms. X-ray diffraction data have been collected to 2.2 Å.
S. cerevisiae Atg3, an E2-like enzyme that mediates the lipidation of Atg8, was crystallized and diffracted to 2.5 Å resolution.
Human p40phox was expressed, purified and crystallized. Diffraction data were collected to a resolution of 3.0 Å.
S. cerevisiae Atg5 in complex with the N-terminal regions of Atg16 was expressed, purified and crystallized in four crystal forms.
Crystals of the recombinant native and selenomethionine PilS protein belong to the orthorhombic space group P21212, with unit-cell parameters a = 77.88, b = 114.53, c = 31.75 Å. The structure will be solved using the MAD method.
The E. coli transcriptional regulator RfaH was cloned, expressed, purified and crystallized and the complex of RfaH with its target DNA oligonucleotide was cocrystallized. Complete diffraction data sets were collected for the apo protein and its nucleic acid complex at 2.4 and at 1.6 Å resolution, respectively.
Pyrrolysyl-tRNA synthetase (PylRS) from M. mazei has been overexpressed in an N-terminally truncated form PylRS(c270) in Escherichia coli, purified to homogeneity and crystallized by the hanging-drop vapour-diffusion method.
L-Methionine γ-lyase 2 from E. histolytica, a key enzyme in sulfur-containing amino-acid degradation in this protozoan parasite, has been crystallized in a form suitable for X-ray structure analysis.
Pig heart carbonyl reductase has been crystallized in the presence of NADPH. Diffraction data have been collected using synchrotron radiation.
Proteolysis in situ by a protease secreted by a contaminating fungus is essential for the crystallization of yeast CPSF-100.
A YaeQ protein from the plant pathogen X. campestris pv. campestris has been overexpressed in E. coli, purified and crystallized. The crystals diffracted well to a resolution of 1.28 Å.
Dihydroorotate dehydrogenase from L. major has been crystallized by the vapour-diffusion technique using lithium sulfate as the precipitant agent. A complete data set from a native crystal has been collected to 2.0 Å resolution using an in-house rotating-anode generator.


 journal menu
journal menu











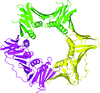
























![[publBio]](http://journals.iucr.org/logos/publbio.gif)





