issue contents
April 2015 issue
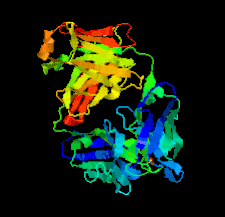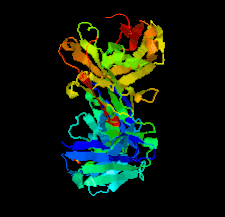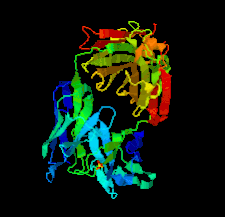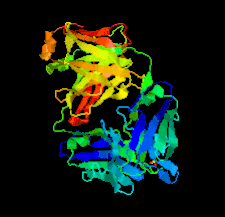
Cover illustration: Structure of the omalizumab Fab (Jensen et al., p. 419).
IYCr crystallization series
Growing large-volume crystals are necessary and important for neutron macromolecular crystallography. Experimental strategies, techniques and theoretical considerations for protein crystal growth are reviewed.
research communications
The crystal structure of human P-cadherin EC1-EC2 in a closed conformation is reported at 1.62 Å resolution, showing a double conformation of Trp2 in the binding pocket and a novel monomeric packing arrangement.
PDB reference: human P-cadherin EC1-EC2, 4oy9
The crystal structure of fructosyl peptide oxidase from E. terrenum was determined at 1.9 Å resolution. This is the first structure of a group I FPOD that prefers α-fructosyl peptide substrates.
PDB reference: fructosyl peptide oxidase, 4rsl
A neutralizing Fab antibody fragment directed against the Wnt antagonist sclerostin was crystallized and its structure was determined by molecular replacement.
In this study, MtaN-1 from A. hydrophila was successfully expressed and purified, and crystals in complex with the substrate SAH were obtained and diffracted to a resolution of 1.4 Å.
A GH12 endoglucanase from A. aculeatus F-50 was expressed in P. pastoris, crystallized and diffracted to a resolution of 1.6 Å.
HutB from V. cholerae has been cloned, overexpressed, purified and crystallized in a monomeric form. Crystals belonging to space group P43212 produced diffraction data to 2.4 Å resolution.
Mutants of Turnip yellow mosaic virus protease/ubiquitin hydrolase designed to prevent self-recognition were produced and crystallized as monomers.
Psb32 from T. elongatus was expressed using a novel vector library for the generation of superfolder fluorescent fusion proteins. X-ray diffraction data were collected to a resolution of 2.3 Å from a crystal belonging to space group P6122.
This crystallization and data collection are reported for a complex of Reb1 bound to Ter3 DNA.
The therapeutic antibody omalizumab inhibits the binding of IgE to its receptors. Two crystal structures of the omalizumab Fab fragment have been determined at 1.9 and 3.0 Å resolution.
V. harveyi β-N-acetylglucosaminidase (VhGlcNAcase) is a glycoside hydrolase that degrades chitooligosacharides, yielding N-acetylglucosamine as the final product. Single crystals of wild-type and D437A-mutant VhGlcNAcase were grown and diffracted to resolutions of 2.37 and 2.60 Å, respectively.
The phosphatase domain PA3346PD of the response regulator PA3346 from P. aeruginosa has been overexpressed, purified and crystallized by the sitting-drop vapour-diffusion method.
Open  access
access
 access
accessThe 800 kDa complex of the human Rod, Zwilch and ZW10 proteins (the RZZ complex) was reconstituted in insect cells, purified, crystallized and subjected to preliminary X-ray diffraction analysis.
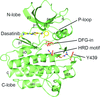
This article reports the co-crystal structure of the mouse ARG catalytic domain with dasatinib, a tyrosine kinase inhibitor used for the treatment of leukemia.
PDB reference: ABL2/ARG kinase in complex with dasatinib, 4xli
The structure of dihydrodipicolinate synthase from B. thetaiotaomicron has been determined. The overall structure is described and compared with related structures.
PDB reference: dihydrodipicolinate synthase, 4xky
Analytical ultracentrifugation studies and crystallization experiments have been performed on the chloroplast envelope quinone oxydoreductase homologue (ceQORH). Diffracting crystals of ceQORH have been obtained and X-ray diffraction data were collected at 2.34 Å resolution.
Four ficin (iso)forms from F. carica latex were isolated and crystallized. For each form, at least one crystal form was obtained that diffracted to a resolution of at least 1.60 Å.
The cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of AerF from M. aeruginosa, which is a putative reductase that participates in the biosynthetic pathway of the 2-carboxy-6-hydroxyoctahydroindole moiety of aeruginosin, are reported.
The cyanate hydratase CynS from S. proteamaculans was crystallized serendipitously during screens for a eukaryotic complex. Here, its crystal structure is described at 2.1 Å resolution.
PDB reference: cyanase, 4y42
The membrane protein NDH-II from the human pathogen S. aureus was produced in E. coli, purified and crystallized. The crystals belonged to the orthorhombic space group P212121 and diffracted to 3.32 Å resolution; the structure was determined by molecular replacement.


 journal menu
journal menu


























![[publBio]](/logos/publbio.gif)