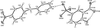issue contents
February 2013 issue
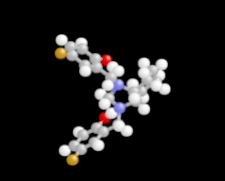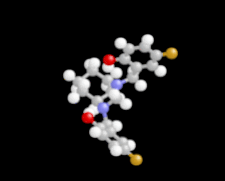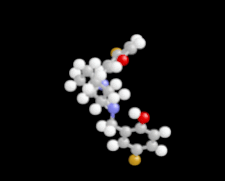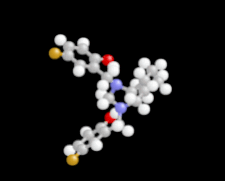
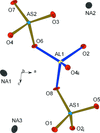
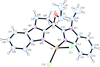
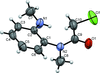
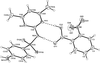
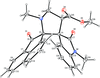
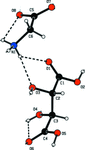
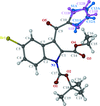
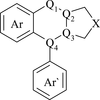
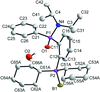
Cover illustration: 1,2-Diamines tend to adopt a conformation in which the arrangement of electron pairs is antiperiplanar. This behaviour is known as the `rabbit-ears effect', however it can be avoided by restriction of the 1,2-diamine in cyclic molecules. The crystal studied, C21H24F2N2O2, was a meso compound obtained by the reaction of the aminal (2S,7R,11S,16R)-1,8,10,17-tetraazapentacyclo[8.8.1.18,17.02,7.011,16]cosane with 4-fluorophenol. The imidazolidine ring has a twisted conformation with the lone pairs of the N atoms disposed in a syn isomerism, making this compound an exception to the typical `rabbit-ears effect' in 1,2-diamines. See: Rivera, Quiroga, Ríos-Motta, Kučeraková & Dušek [Acta Cryst. (2013). E69, o217].
inorganic compounds
















metal-organic compounds






















































































organic compounds

























































































































































































































































































 journal menu
journal menu